Alle Fotos(1)
Wichtige Dokumente
214930
1,2,3,4-Tetrahydro-3-isochinolincarbonsäure -hydrochlorid
96%
Anmeldenzur Ansicht organisationsspezifischer und vertraglich vereinbarter Preise
Alle Fotos(1)
About This Item
Empirische Formel (Hill-System):
C10H11NO2 · HCl
CAS-Nummer:
Molekulargewicht:
213.66
Beilstein:
3723332
EG-Nummer:
MDL-Nummer:
UNSPSC-Code:
12352100
PubChem Substanz-ID:
NACRES:
NA.22
Empfohlene Produkte
Qualitätsniveau
Assay
96%
mp (Schmelzpunkt)
>300 °C (lit.)
SMILES String
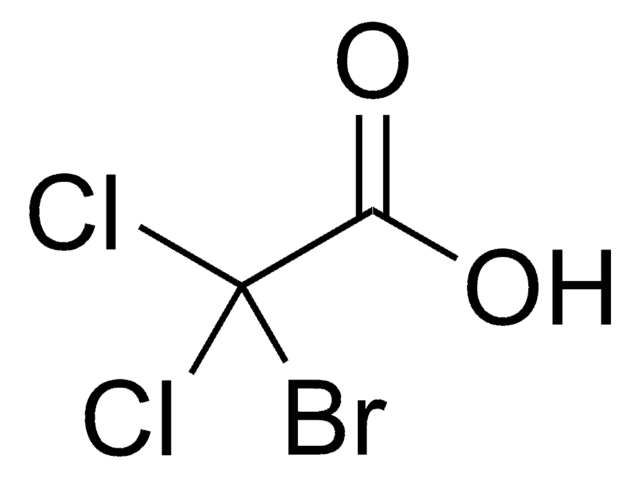
Cl[H].OC(=O)C1Cc2ccccc2CN1
InChI
1S/C10H11NO2.ClH/c12-10(13)9-5-7-3-1-2-4-8(7)6-11-9;/h1-4,9,11H,5-6H2,(H,12,13);1H
InChIKey
FXHCFPUEIDRTMR-UHFFFAOYSA-N
Suchen Sie nach ähnlichen Produkten? Aufrufen Leitfaden zum Produktvergleich
Anwendung
1,2,3,4-Tetrahydro-3-isoquinolinecarboxylic acid was used in the synthesis of 10,10a-dihydroimidazo-[1,5-b]isoquinoline-1,3(2H,5H)-diones, inhibitor of inflammation, apoprotein B-100 biosynthesis and matrix-degrading metalloprotienase.
Signalwort
Warning
H-Sätze
Gefahreneinstufungen
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Zielorgane
Respiratory system
Lagerklassenschlüssel
11 - Combustible Solids
WGK
WGK 3
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Persönliche Schutzausrüstung
dust mask type N95 (US), Eyeshields, Gloves
Hier finden Sie alle aktuellen Versionen:
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Alan R Katritzky et al.
The Journal of organic chemistry, 67(23), 8224-8229 (2002-11-09)
Condensations of chiral diamines 11a-c with benzotriazole and formaldehyde gave benzotriazolyl intermediates 12a-c; similar condensations of alpha-amino-amides 10a-c with benzotriazole and paraformaldehyde gave 14a-c. Subsequent treatment of 12a-c and 14a-c with AlCl(3) led to enantiopure tricyclic 1,2,3,5,10,10a-hexahydroimidazo[1,5-b]isoquinolines 1a-c and 2,3,10,10a-tetrahydroimidazo[1,5-b]isoquinolin-1(5H)-ones
Raman K Bakshi et al.
Bioorganic & medicinal chemistry letters, 15(14), 3430-3433 (2005-06-14)
The discovery of 1-amino-1,2,3,4-tetrahydronaphthalene-2-carboxylic acid analogs as potent human melanocortin-4 selective agonists is described.
B C Wilkes et al.
Biopolymers, 34(9), 1213-1219 (1994-09-01)
A molecular mechanics study (grid search and energy minimization) of the highly delta receptor-selective delta opioid antagonist H-Tyr-Tic-Phe-OH (TIP; Tic: tetrahydroisoquinoline-3-carboxylic acid) resulted in four low energy conformers with energies within 2 kcal/mol of that of the lowest energy structure.
M Manning et al.
Journal of peptide science : an official publication of the European Peptide Society, 1(1), 66-79 (1995-01-01)
We have investigated the effects of mono-substitutions with the conformationally restricted amino acid, 1,2,3,4 tetrahydroisoquinoline-3-carboxylic acid (Tic) at position 3 in arginine vasopressin (AVP), at positions 2, 3 and 7 in potent non-selective cyclic AVP V2/V1a antagonists, in potent and
Yingjie Zhang et al.
Current protein & peptide science, 11(8), 752-758 (2011-01-18)
Tic, short for 1,2,3,4-tetrahydroisoquinoline-3-carboxylic acid, is a kind of unnatural α-amino acids. Due to its distinct geometrical conformation and biological activity, the structure of Tic, regarded as the surrogate of proline and the rigid analogue of phenylalanine or tyrosine, has
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.