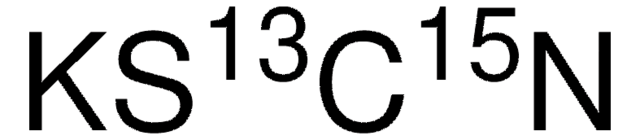Alle Fotos(2)
Wichtige Dokumente
167134
Bromhydrochinon
97%
Anmeldenzur Ansicht organisationsspezifischer und vertraglich vereinbarter Preise
Alle Fotos(2)
About This Item
Lineare Formel:
BrC6H3(OH)2
CAS-Nummer:
Molekulargewicht:
189.01
EG-Nummer:
MDL-Nummer:
UNSPSC-Code:
12352100
PubChem Substanz-ID:
NACRES:
NA.22
Empfohlene Produkte
Assay
97%
mp (Schmelzpunkt)
112-116 °C (lit.)
Funktionelle Gruppe
bromo
SMILES String
Oc1ccc(O)c(Br)c1
InChI
1S/C6H5BrO2/c7-5-3-4(8)1-2-6(5)9/h1-3,8-9H
InChIKey
REFDOIWRJDGBHY-UHFFFAOYSA-N
Verwandte Kategorien
Anwendung
Bromohydroquinone was used in the synthesis of Π-conjugated polymers composed of alkyl carbazole/dialkoxyphenylene and squaraine units via Sonogashira cross-coupling reactions. It was used in the preparation of 2-bromobenzoquinone.
Signalwort
Warning
H-Sätze
Gefahreneinstufungen
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Zielorgane
Respiratory system
Lagerklassenschlüssel
11 - Combustible Solids
WGK
WGK 3
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Persönliche Schutzausrüstung
dust mask type N95 (US), Eyeshields, Gloves
Hier finden Sie alle aktuellen Versionen:
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
T J Monks et al.
Molecular pharmacology, 34(1), 15-22 (1988-07-01)
The formation of potentially reactive thiols has been postulated to play a role in the nephrotoxicity caused by a number of glutathione and/or cysteine conjugates. However, the inherent reactivity of such compounds has precluded both their identification in biological systems
T J Monks et al.
Drug metabolism and disposition: the biological fate of chemicals, 13(5), 553-559 (1985-09-01)
Incubation of either o-bromophenol or 2-bromohydroquinone with rat liver microsomes and 0.25 mM 35S-glutathione (GSH) gave rise to several isomeric 35S-GSH conjugates. A mixture of these isomeric GSH conjugates was prepared chemically and two were purified by HPLC; 1H-NMR spectroscopy
R G Schnellmann
Toxicology and applied pharmacology, 99(1), 11-18 (1989-06-01)
2-Bromohydroquinone (BHQ) plays an important role in bromobenzene-induced nephrotoxicity and is a model toxic hydroquinone. Since BHQ has a quinone nucleus and various quinones have been shown to produce cytotoxicity via oxidative stress, the goal of this study was to
G W Miller et al.
Toxicology and applied pharmacology, 125(2), 192-197 (1994-04-01)
We have previously shown that strychnine mimics the cytoprotective properties of glycine in renal proximal tubule (RPT) suspensions exposed to antimycin A (AA). The aims of this study were to determine whether the cytoprotective properties of strychnine applied to various
S S Lau et al.
Biochemical and biophysical research communications, 152(1), 223-230 (1988-04-15)
2-Bromo-(diglutathion-Syl)hydroquinone (2-Br-[diGSyl]HQ) is a more potent nephrotoxicant than any of three mono-substituted isomers. The reason for this differential toxicity is unknown. We now report that the rate of uptake of 2-Br-(diGSyl)HQ, 2-Br-3-(GSyl)HQ, 2-Br-5-(GSyl)-HQ and 2-Br-6(GSyl)HQ by kidney slices was 2.4
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.