121231
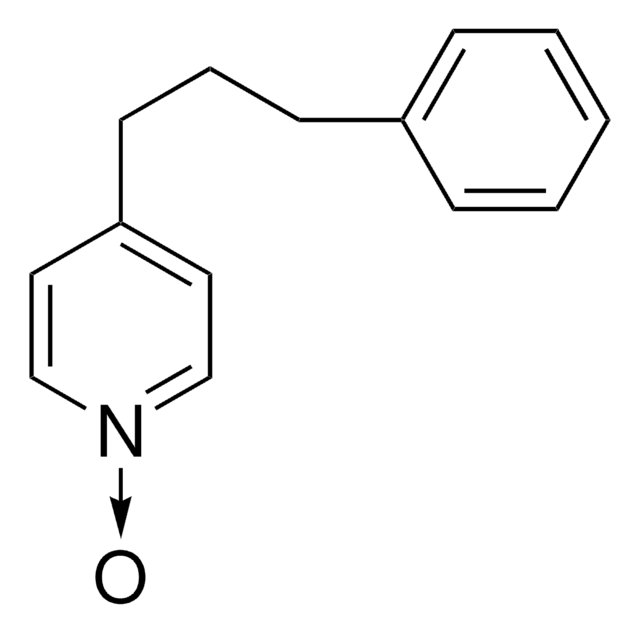
4-(3-Phenylpropyl)pyridin
97%
Anmeldenzur Ansicht organisationsspezifischer und vertraglich vereinbarter Preise
Alle Fotos(1)
About This Item
Empirische Formel (Hill-System):
C14H15N
CAS-Nummer:
Molekulargewicht:
197.28
EG-Nummer:
MDL-Nummer:
UNSPSC-Code:
12352100
eCl@ss:
39151717
PubChem Substanz-ID:
NACRES:
NA.22
Empfohlene Produkte
Qualitätsniveau
Assay
97%
Brechungsindex
n20/D 1.563 (lit.)
bp
322 °C (lit.)
Löslichkeit
H2O: insoluble
Dichte
1.03 g/mL at 25 °C (lit.)
Funktionelle Gruppe
phenyl
SMILES String
C(Cc1ccccc1)Cc2ccncc2
InChI
1S/C14H15N/c1-2-5-13(6-3-1)7-4-8-14-9-11-15-12-10-14/h1-3,5-6,9-12H,4,7-8H2
InChIKey
AQIIVEISJBBUCR-UHFFFAOYSA-N
Allgemeine Beschreibung
4-(3-Phenylpropyl) pyridine is an organic solvent and is a suitable medium for water insoluble transition metal complexes of porphyrins or phthalocyanines.
Anwendung
4-(3-Phenylpropyl) pyridine(PPP) was used in electrochemical processes for a novel system of microdroplets of PPP immobilised at graphite and in mesoporous ceramic electrodes. PPP has been used to investigate electrochemically driven mono-, di-, and tri-carboxylate anion transfer at the 4-(3-phenylpropyl)pyridine/aqueous electrolyte interface for a triple phase boundary system at graphite electrodes.
Signalwort
Warning
H-Sätze
Gefahreneinstufungen
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Zielorgane
Respiratory system
Lagerklassenschlüssel
10 - Combustible liquids
WGK
WGK 3
Flammpunkt (°F)
235.4 °F - closed cup
Flammpunkt (°C)
113 °C - closed cup
Persönliche Schutzausrüstung
Eyeshields, Gloves, type ABEK (EN14387) respirator filter
Hier finden Sie alle aktuellen Versionen:
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Frank Marken et al.
Faraday discussions, 129, 219-229 (2005-02-18)
Biphasic electrode systems allow electrochemical reactions to be driven in a microphase of organic liquid (typically 1-100 nL), which is coupled via ion transfer processes to the surrounding aqueous electrolyte medium. Microdroplet deposits on basal plane pyrolytic graphite as well
Stuart M MacDonald et al.
Physical chemistry chemical physics : PCCP, 10(26), 3925-3933 (2008-08-09)
Understanding liquid|liquid ion transfer processes is important in particular for naturally occurring species such as carboxylates. In this study electrochemically driven mono-, di-, and tri-carboxylate anion transfer at the 4-(3-phenylpropyl)pyridine|aqueous electrolyte interface is investigated experimentally for a triple phase boundary
Xueqiang Qi et al.
Dalton transactions (Cambridge, England : 2003), 43(24), 9283-9295 (2014-05-14)
Core-shell RuPt (Ru core-Pt shell) and PtRu (Pt core-Ru shell) nanoparticles were prepared by decomposing in a two-step procedure a ruthenium ([Ru(COD)(COT)] (COD = 1,5-cyclooctadiene, COT = 1,3,5-cyclooctatriene)) and a platinum complex ([Pt2(dba)3] (dba = dibenzylideneacetone) or [Pt(CH3)2(COD)]) in the
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.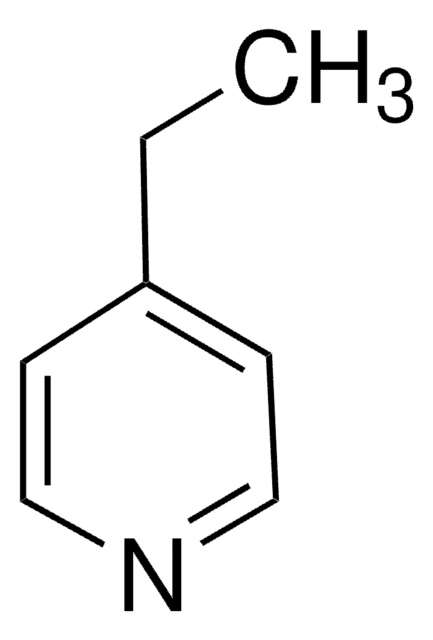
![4-[6-(4-pyridinyl)hexyl]pyridine AldrichCPR](/deepweb/assets/sigmaaldrich/product/structures/969/304/69102a98-4497-4db9-b5d7-792b9c6fb5e7/640/69102a98-4497-4db9-b5d7-792b9c6fb5e7.png)