Alle Fotos(1)
Wichtige Dokumente
104981
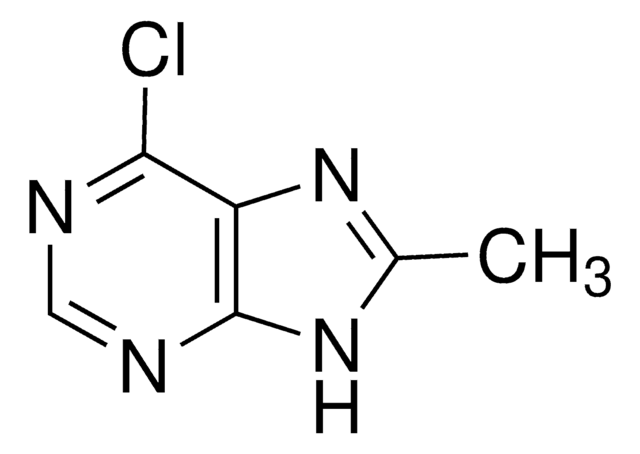
6-Brompurin
98%
Anmeldenzur Ansicht organisationsspezifischer und vertraglich vereinbarter Preise
Alle Fotos(1)
About This Item
Empirische Formel (Hill-System):
C5H3BrN4
CAS-Nummer:
Molekulargewicht:
199.01
EG-Nummer:
MDL-Nummer:
UNSPSC-Code:
12352100
PubChem Substanz-ID:
NACRES:
NA.22
Empfohlene Produkte
Qualitätsniveau
Assay
98%
Form
solid
mp (Schmelzpunkt)
>300 °C (lit.)
Funktionelle Gruppe
bromo
SMILES String
Brc1ncnc2nc[nH]c12
InChI
1S/C5H3BrN4/c6-4-3-5(9-1-7-3)10-2-8-4/h1-2H,(H,7,8,9,10)
InChIKey
CTGFGRDVWBZYNB-UHFFFAOYSA-N
Verwandte Kategorien
Allgemeine Beschreibung
6-Bromopurine enhances the carcinostatic activity of azaserine in a test system employing ascites cell forms of sarcoma 180 and Ehrlich carcinoma in vivo. 6-bromopurine nucleosides are excellent substrates for substitution reactions with N-, O-, and S-containing nucleophiles in polar solvents.
Anwendung
6-Bromopurine was used in the synthesis of 6-halopurine alkynes and corresponding triazole derivatives. 6-Bromopurine was used in the synthesis and chemical characterization of 2,3,4,5-tetrahydro-1,5-benzoxazepines-3-ol.
Signalwort
Warning
H-Sätze
Gefahreneinstufungen
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Zielorgane
Respiratory system
Lagerklassenschlüssel
11 - Combustible Solids
WGK
WGK 3
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Persönliche Schutzausrüstung
dust mask type N95 (US), Eyeshields, Gloves
Hier finden Sie alle aktuellen Versionen:
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Comparison of some biologgical and biochemical properties of 6-bromopurine and 6-iodopurine.
A C SARTORELLI et al.
Biochemical pharmacology, 11, 1017-1024 (1962-11-01)
E A Véliz et al.
The Journal of organic chemistry, 66(25), 8592-8598 (2001-12-12)
Surprisingly facile direct substitution reactions with acetyl-protected 6-bromopurine nucleosides are described. Included in the series of bromonucleosides studied is the guanosine derivative N(2)-2',3',5'-tetraacetyl-6-bromopurine ribonucleoside, the synthesis of which is reported here for the first time. Brominated nucleosides had not previously
Synthesis, unambiguous chemical characterization, and reactivity of 2, 3, 4, 5-tetrahydro-1, 5-benzoxazepines-3-ol.
Garcia-Rubino ME, et al.
Royal Society of Chemistry Advances, 2(33), 12631-12635 (2012)
Eva Galante et al.
Molecules (Basel, Switzerland), 18(5), 5335-5347 (2013-05-15)
2-[¹⁸F]Fluoroethyl azide ([¹⁸F]FEA) can readily be obtained by nucleophilic substitution of 2-azidoethyl-4-toluenesulfonate with [¹⁸F]fluoride (half-life 110 min), and has become widely used as a reagent for 'click' labeling of PET tracers. However, distillation of [18F]FEA is typically required, which is
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.
![2,4-Dichloro-7H-pyrrolo[2,3-d]pyrimidine-7-carboxylic acid tert-butyl ester AldrichCPR](/deepweb/assets/sigmaaldrich/product/structures/315/036/b807f57a-439c-4114-b92d-077d429f82f3/640/b807f57a-439c-4114-b92d-077d429f82f3.png)
