1A00900
USP
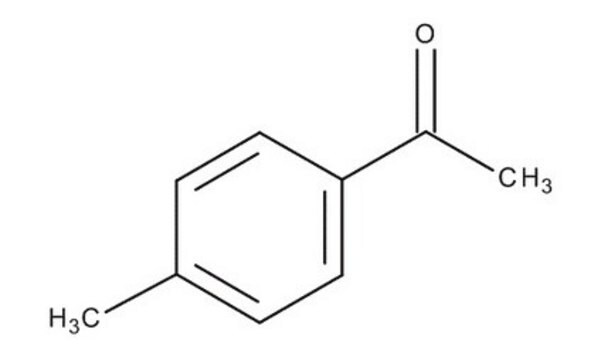
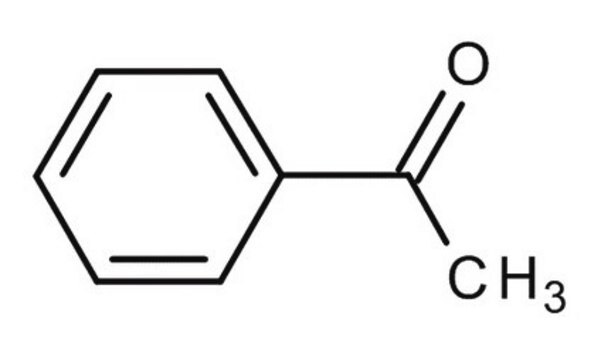
4-Methylacetophenone
Pharmaceutical Analytical Impurity (PAI)
Synonyme(s) :
(1-(4-Methylphenyl)-1-ethanone (4′-Methylacetophenone))
Se connecterpour consulter vos tarifs contractuels et ceux de votre entreprise/organisme
About This Item
Formule empirique (notation de Hill):
C9H10O
Numéro CAS:
Poids moléculaire :
134.18
Code UNSPSC :
41116100
Produits recommandés
Qualité
pharmaceutical analytical impurity (PAI)
Agence
USP
Fabricant/nom de marque
USP
Application(s)
pharmaceutical
Format
neat
Température de stockage
2-8°C
Catégories apparentées
Description générale
4-Methylacetophenone is a USP Pharmaceutical Analytical Impurity (PAI).
USP PAI are a product line of impurities suitable for research and analytical purposes, which help to ensure the quality and safety of medicines.
Associated Drug Substance: Celecoxib
Therapeutic Area: Analgesics
For more information about this PAI, visit here.
USP PAI are a product line of impurities suitable for research and analytical purposes, which help to ensure the quality and safety of medicines.
Associated Drug Substance: Celecoxib
Therapeutic Area: Analgesics
For more information about this PAI, visit here.
Application
4-Methylacetophenone (USP PAI) is intended for use in analytical testing to detect, identify, and measure pharmaceutical impurities.
Caractéristiques et avantages
USP PAI advance your early analytical R&D and process development. PAI can be used in the following applications:
1. Conduct analytical tests during early formulation feasibility studies.
2. Determine degradation impurities produced during stress studies.
3. Develop, validate, and transfer analytical methods.
4. Perform spiking studies during process R&D to demonstrate depletion upon recrystallization.
5. Record retention times and/or spectra
6. Determine relative response factors.
7. Identify unknown impurities that formed during ICH stability conditions.
8. Identify impurities that are present in the Reference Listed Drug
9. Test for and profile impurities not listed in drug substance and drug product monographs.
1. Conduct analytical tests during early formulation feasibility studies.
2. Determine degradation impurities produced during stress studies.
3. Develop, validate, and transfer analytical methods.
4. Perform spiking studies during process R&D to demonstrate depletion upon recrystallization.
5. Record retention times and/or spectra
6. Determine relative response factors.
7. Identify unknown impurities that formed during ICH stability conditions.
8. Identify impurities that are present in the Reference Listed Drug
9. Test for and profile impurities not listed in drug substance and drug product monographs.
Remarque sur l'analyse
These products are for test and assay use only. They are not meant for administration to humans or animals and cannot be used to diagnose, treat, or cure diseases of any kind.
Autres remarques
Sales restrictions may apply.
Mention d'avertissement
Warning
Mentions de danger
Conseils de prudence
Classification des risques
Acute Tox. 4 Oral - Skin Irrit. 2
Code de la classe de stockage
10 - Combustible liquids
Classe de danger pour l'eau (WGK)
WGK 1
Certificats d'analyse (COA)
Recherchez un Certificats d'analyse (COA) en saisissant le numéro de lot du produit. Les numéros de lot figurent sur l'étiquette du produit après les mots "Lot" ou "Batch".
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique