1494909
USP
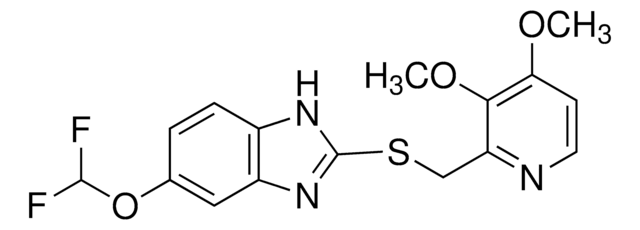
Pantoprazole Related Compound A
United States Pharmacopeia (USP) Reference Standard
Synonyme(s) :
Pantoprazole sulfone, 5-(Difluoromethoxy)-2-{[(3,4-dimethoxy-2-pyridinyl)methyl]sulfone}-1H-benzimidazole
About This Item
Produits recommandés
Qualité
pharmaceutical primary standard
Famille d'API
pantoprazole
Fabricant/nom de marque
USP
Application(s)
pharmaceutical (small molecule)
Format
neat
InChI
1S/C16H15F2N3O5S/c1-24-13-5-6-19-12(14(13)25-2)8-27(22,23)16-20-10-4-3-9(26-15(17)18)7-11(10)21-16/h3-7,15H,8H2,1-2H3,(H,20,21)
Clé InChI
FCJYMBZQIJDMMM-UHFFFAOYSA-N
Vous recherchez des produits similaires ? Visite Guide de comparaison des produits
Application
- Transcriptional profiling for drug repurposing in glioblastoma: This study utilized transcriptional profiling to identify therapeutic targets and repurpose drugs, including pantoprazole-related compounds, for treatment in glioblastoma. This approach underscores the utility of pantoprazole impurities in exploring new therapeutic avenues in pharmaceutical reference material research (Roddy et al., 2023).
- Gastroprotective agent utilization: A drug utilization study in medicine and surgery wards highlighted the significant role of pantoprazole and its related compounds in managing gastrointestinal protection. This research emphasizes the importance of pantoprazole as a proton pump inhibitor and its impurities in ensuring the efficacy and safety of gastroprotective therapies (Koyani et al., 2023).
- Synthesis and analysis of pantoprazole sodium sesquihydrate-related compound E: This study explored the synthesis and mechanism of formation of a specific pantoprazole-related compound, offering insights into the chemical stability and quality control of pantoprazole as a pharmaceutical ingredient. Such research is crucial for enhancing analytical methods in pharmaceutical research and ensuring drug safety and efficacy (Yari et al., 2019).
Remarque sur l'analyse
Autres remarques
Produit(s) apparenté(s)
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Certificats d'analyse (COA)
Recherchez un Certificats d'analyse (COA) en saisissant le numéro de lot du produit. Les numéros de lot figurent sur l'étiquette du produit après les mots "Lot" ou "Batch".
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique