1066009
USP
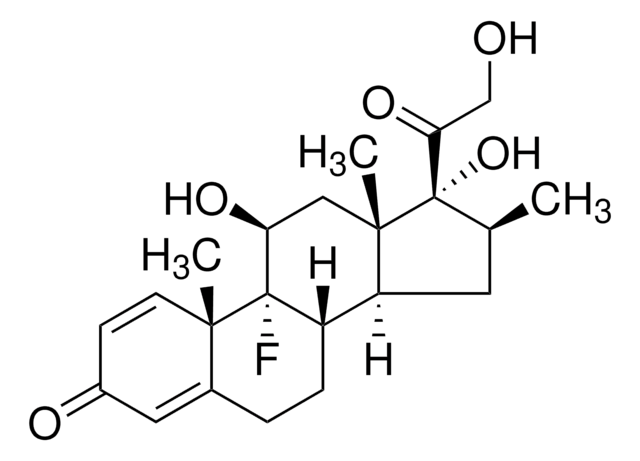
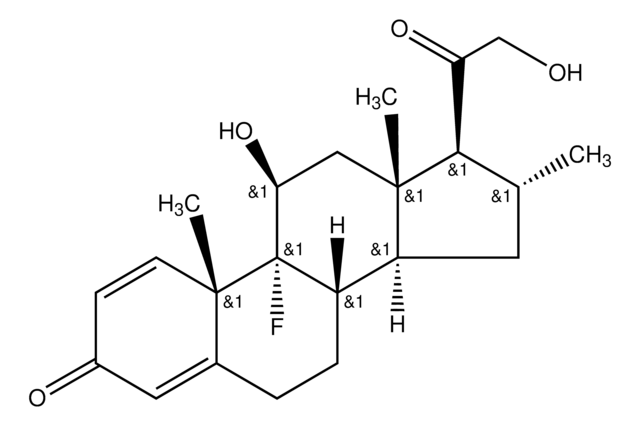
Betamethasone
United States Pharmacopeia (USP) Reference Standard
Synonyme(s) :
9α-Fluoro-11β,17α,21-trihydroxy-16β-methylpregna-1,4-diene-3,20-dione, 9α-Fluoro-16β-methyl-11β,17α,21-trihydroxy-1,4-pregnadiene-3,20-dione, 9α-Fluoro-16β-methylprednisolone
About This Item
Produits recommandés
Source biologique
synthetic
Qualité
pharmaceutical primary standard
Agence
USP
Pression de vapeur
<0.0000001 kPa ( 25 °C)
Famille d'API
betamethasone
Conditionnement
pkg of 200 mg
Fabricant/nom de marque
USP
Couleur
white to off-white
Pf
235-237 °C (lit.)
447.8-473 °F (231—245°C; decomposes)
Solubilité
acetone: sparingly soluble
chloroform: very slightly soluble
ethanol: sparingly soluble
ether: very slightly soluble
methanol: sparingly soluble
water: insoluble
Densité
0.305 g/cm3 at 25 °C (77°F)
Application(s)
pharmaceutical (small molecule)
Format
neat
Température de stockage
2-8°C
Chaîne SMILES
[H][C@@]12C[C@H](C)[C@](O)(C(=O)CO)[C@@]1(C)C[C@H](O)[C@@]3(F)[C@@]2([H])CCC4=CC(=O)C=C[C@]34C
InChI
1S/C22H29FO5/c1-12-8-16-15-5-4-13-9-14(25)6-7-19(13,2)21(15,23)17(26)10-20(16,3)22(12,28)18(27)11-24/h6-7,9,12,15-17,24,26,28H,4-5,8,10-11H2,1-3H3/t12-,15-,16-,17-,19-,20-,21-,22-/m0/s1
Clé InChI
UREBDLICKHMUKA-DVTGEIKXSA-N
Informations sur le gène
human ... NR3C1(2908)
Vous recherchez des produits similaires ? Visite Guide de comparaison des produits
Description générale
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the issuing Pharmacopoeia.For further information and support please go to the website of the issuing Pharmacopoeia.
Application
- Betamethasone Acetate
- Betamethasone Oral Solution
- Betamethasone Valerate Cream
- Betamethasone Valerate Lotion
- Betamethasone Valerate Ointment
- Dexamethasone
Remarque sur l'analyse
Autres remarques
Produit(s) apparenté(s)
Mention d'avertissement
Danger
Mentions de danger
Conseils de prudence
Classification des risques
Repr. 1B - STOT RE 2
Organes cibles
Liver,Kidney,Endocrine system
Code de la classe de stockage
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
Classe de danger pour l'eau (WGK)
WGK 2
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Faites votre choix parmi les versions les plus récentes :
Certificats d'analyse (COA)
It looks like we've run into a problem, but you can still download Certificates of Analysis from our Documents section.
Si vous avez besoin d'assistance, veuillez contacter Service Clients
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Protocoles
A simple, precise and sensitive Reverse-Phase High Pressure Liquid Chromatography gradient method was adapted for traceability, homogeneity and total chromatographic analysis of Dexamethasone. The given experimental conditions follow the USP43-NF38 monograph method for Dexamethasone Assay and Organic Impurity Profiling. Dexamethasone, Betamethasone, Dexamethasone acetate and Desoximetasone were baseline resolved within 20 minutes using a Titan C18 UHPLC column (2.1 x 100 mm, 1.9 µm).
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique