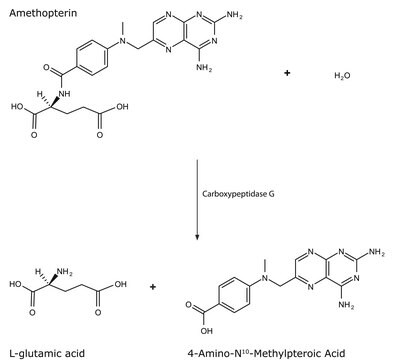
SAE0046
Carboxypeptidase A from bovine pancreas
(Type II-PMSF treated), ≥20 units/mg protein
Synonyme(s) :
Carboxypeptidase A, Carboxypolypeptidase, Peptidyl-L-amino-acid hydrolase
About This Item
Produits recommandés
Source biologique
bovine pancreas
Niveau de qualité
Forme
aqueous suspension
Qualité
(Type II-PMSF treated)
Activité spécifique
≥20 units/mg protein
Poids mol.
~35 kDa
Produit purifié par
2× crystallization
Impuretés
≤0.05 BTEE units/mg protein chymotrpsin
≤10 BAEE units/mg protein trypsin
Température de stockage
2-8°C
Vous recherchez des produits similaires ? Visite Guide de comparaison des produits
Catégories apparentées
Description générale
Application
Actions biochimiques/physiologiques
Définition de l'unité
Notes préparatoires
Remarque sur l'analyse
Certificats d'analyse (COA)
Recherchez un Certificats d'analyse (COA) en saisissant le numéro de lot du produit. Les numéros de lot figurent sur l'étiquette du produit après les mots "Lot" ou "Batch".
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique