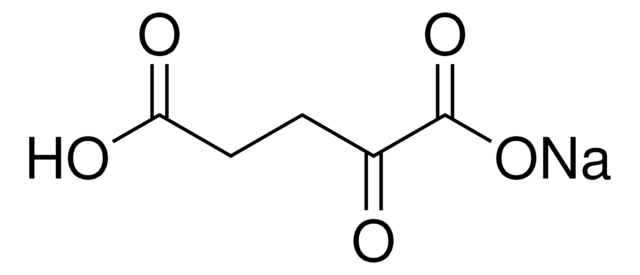
P4591
Pyruvate Oxidase from microorganisms
lyophilized powder, ≥1.5 U/mg
Synonyme(s) :
Pyruvate:oxygen oxidoreductase (phosphorylating)
Se connecterpour consulter vos tarifs contractuels et ceux de votre entreprise/organisme
About This Item
Produits recommandés
Forme
lyophilized powder
Niveau de qualité
Activité spécifique
≥1.5 U/mg
Poids mol.
~260 kDa
Température de stockage
−20°C
Application
Pyruvate Oxidase (PoxB) converts pyruvate directly to acetate and CO2. It is used to study pyruvate metabolism. It is used to study aerobic metabolism of bacterium, such as Lactobacillus plantarumand Streptococcus pneumoniae. Pyruvate Oxidase is used for enzymatic determination of pyruvate, GOT, and GPT in clinical analysis.
Pyruvate Oxidase from microorganisms has been used in the Amplex Red-based fluorescence assay for pyruvate. It has also been used in developing near real-time continuous detection system for pyruvate.
Actions biochimiques/physiologiques
Pyruvate Oxidase consists of four subunits with identical molecular weights. PoxB reacts with certain aldehydes and phosphate can be replaced by arsenate. Oxygen as well as several artificial compounds can function as electron acceptors. Pyruvate Oxidase is activated by phospholipids as well as monomeric and micellar amphiphiles.
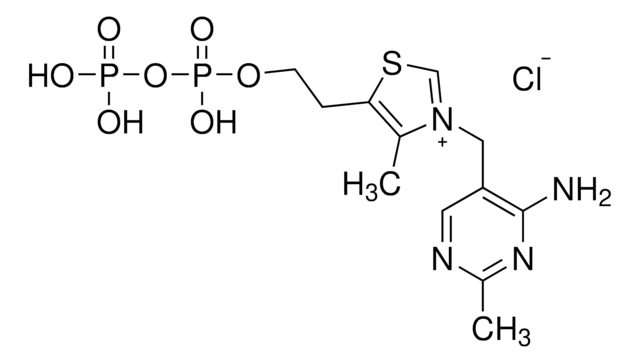
Pyruvate Oxidase oxidises pyruvate to form acetate, hydrogen peroxide and carbondioxide. Pyruvate oxidase requires flavin adenine dinucleotide (FAD), thiamine pyrophosphate (TPP) and magnesium as cofactors for its catalytic activity. Pyruvate oxidase is activated by the cofactor thiamine.
Propriétés physiques
Isoelectric point : 4.3
Michaelis constant : 3.4 X 10-4M (Pyruvate)
Inhibitors : Fe++,Zn++,Cu++,Ag+,Hg++
Optimum pH : 5.7
Optimum temperature : 65oC
pH Stability : pH 5.7 - 6.5 (25oC, 20hr)
Thermal stability : below 45oC (pH 6.0, 15min)
Michaelis constant : 3.4 X 10-4M (Pyruvate)
Inhibitors : Fe++,Zn++,Cu++,Ag+,Hg++
Optimum pH : 5.7
Optimum temperature : 65oC
pH Stability : pH 5.7 - 6.5 (25oC, 20hr)
Thermal stability : below 45oC (pH 6.0, 15min)
Définition de l'unité
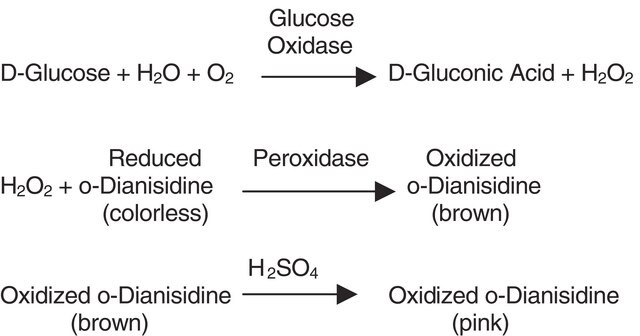
One unit will produce 1.0 μmole of H2O2 per min during the conversion of pyruvate and phosphate to acetylphosphate and CO2 at pH 5.7 at 37 °C.
Forme physique
Lyophilized powder containing FAD and sugar as stabilizer
Mention d'avertissement
Danger
Mentions de danger
Conseils de prudence
Classification des risques
Resp. Sens. 1
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Équipement de protection individuelle
Eyeshields, Gloves, type N95 (US)
Certificats d'analyse (COA)
Recherchez un Certificats d'analyse (COA) en saisissant le numéro de lot du produit. Les numéros de lot figurent sur l'étiquette du produit après les mots "Lot" ou "Batch".
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Multiplexed and fully automated detection of metabolic biomarkers using microdialysis probe
Das C, et al.
Sensors and Actuators B, Chemical, 238, 633-640 (2017)
A sensitive fluorimetric assay for pyruvate
Zhu A, et al.
Analytical Biochemistry, 396(1), 146-151 (2010)
Lan-yan Zheng et al.
International journal of oral science, 3(2), 82-89 (2011-04-13)
The objective of this study was to characterize the oxygen dependent regulation of pyruvate oxidase (SpxB) gene expression and protein production in Streptococcus sanguinis (S. sanguinis). SpxB is responsible for the generation of growth-inhibiting amounts of hydrogen peroxide (H2O2) able
Amit Priyadarshi et al.
Biochemical and biophysical research communications, 380(4), 797-801 (2009-04-03)
MenD (2-succinyl-5-enolpyruvyl-6-hydroxy-3-cyclohexadiene-1-carboxylate) synthase belongs to the superfamily of thiamin diphosphate-dependent decarboxylases, which converts isochorismate and 2-oxoglutarate to SHCHC, pyruvate, and carbon dioxide. Here, we report the first crystal structure of apo-MenD from Escherichia coli determined in tetragonal crystal form. The
Rachel Benisty et al.
Biochimica et biophysica acta, 1801(9), 1098-1104 (2010-07-06)
FabF elongation condensing enzyme is a critical factor in determining the spectrum of products produced by the FASII pathway. Its active site contains a critical cysteine-thiol residue, which is a plausible target for oxidation by H2O2. Streptococcus pneumoniae produces exceptionally
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique