P0515
Phospholipase D from Arachis hypogaea (peanut)
Type II, lyophilized powder, ≥60 units/mg protein
Synonyme(s) :
Lecithinase D, Phosphatidylcholine phosphatidohydrolase
Se connecterpour consulter vos tarifs contractuels et ceux de votre entreprise/organisme
About This Item
Produits recommandés
Source biologique
Arachis hypogaea
Niveau de qualité
Type
Type II
Forme
lyophilized powder
Activité spécifique
≥60 units/mg protein
Composition
Protein, ~30%
Température de stockage
−20°C
Vous recherchez des produits similaires ? Visite Guide de comparaison des produits
Description générale
Phospholipase D is a phospholipid hydrolyzing enzyme and an important component of receptor-mediated signal transduction responses and regulated secretion.
Phospholipase D (PLD) is abundantly found in the eukaryotes and prokaryotes. It is present in all mammalian cells. In mammals, PLD is initiated by various hormones, neurotransmitters, growth factors and cytokines.
Application
Phospholipase D from Arachis hypogaea (peanut) has been used in the preparation of lipase enzyme solution. It has also been used to determine its influence on human neutrophil respiratory function.
Research has shown ADP-ribosylation factor regulation of phospholipase D is important in the release of nascent secretory vesicles from the trans-Golgi network. It has also been used in a study to investigate stimulation of Na+-Ca2+ exchange activity in canine cardiac sarcolemmal vesicals.
Actions biochimiques/physiologiques
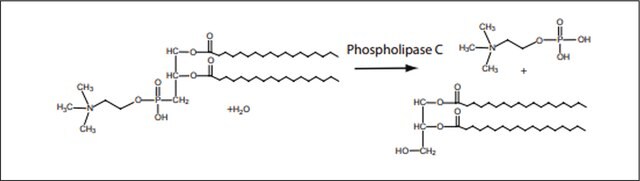
Hydrolyzes the phosphate bonds of phospholipids and sphingomyelin to give the corresponding phosphatidic acid.
Phospholipase D is involved in conferring drought susceptibility in peanuts, which increases the risk of aflatoxin contamination.
Phospholipase D (PLD) modulates cell growth, secretion and the actin cytoskeleton.
Définition de l'unité
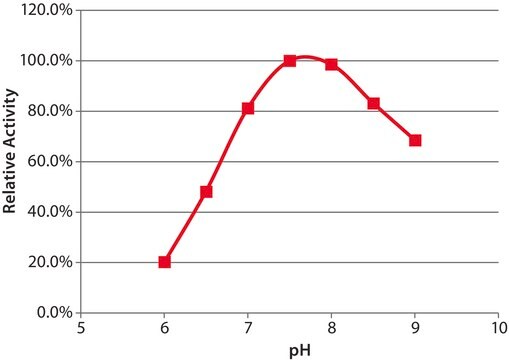
One unit will liberate 1.0 μmol of choline from L-α-phosphatidylcholine (egg yolk) per hr at pH 5.6 at 30 °C.
Forme physique
Partially purified, lyophilized powder containing buffer salts
Remarque sur l'analyse
Protein determined using biuret, unless otherwise indicated.
Inhibiteur
Réf. du produit
Description
Tarif
Mention d'avertissement
Danger
Mentions de danger
Conseils de prudence
Classification des risques
Resp. Sens. 1
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 3
Équipement de protection individuelle
Eyeshields, Gloves, type N95 (US)
Faites votre choix parmi les versions les plus récentes :
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
B Z Guo et al.
Planta, 223(3), 512-520 (2005-10-04)
Preharvest aflatoxin contamination has been identified by the peanut industry as a serious issue in food safety and human health because of the carcinogenic toxicity. Drought stress is the most important environmental factor exacerbating Aspergillus infection and aflatoxin contamination in
K D Philipson et al.
The Journal of biological chemistry, 259(1), 16-19 (1984-01-10)
Treatment of canine cardiac sarcolemmal vesicles with phospholipase D resulted in a large stimulation (up to 400%) of Na+-Ca2+ exchange activity. The phospholipase D treatment decreased the apparent Km (Ca2+) for the initial rate of Nai+-dependent Ca2+ uptake from 18.2
Human neutrophil peptide receptors: mobilization mediated by phospholipase C.
Nelson R D, et al.
The American Journal of Pathology, 107(2), 202-202 (1982)
Expression and characterization of rat brain phospholipase D
Xie Z, et al.
Methods in Enzymology, 345, 255-264 (2002)
Giuseppe Dionisio et al.
Plant physiology, 156(3), 1087-1100 (2011-01-12)
Barley (Hordeum vulgare) and wheat (Triticum aestivum) possess significant phytase activity in the mature grains. Maize (Zea mays) and rice (Oryza sativa) possess little or virtually no preformed phytase activity in the mature grain and depend fully on de novo
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique