O9515
Prolyl oligopeptidase
recombinant, expressed in E. coli
Synonyme(s) :
Prolyl endopeptidase
Se connecterpour consulter vos tarifs contractuels et ceux de votre entreprise/organisme
About This Item
Code UNSPSC :
12352204
Nomenclature NACRES :
NA.54
Produits recommandés
Produit recombinant
expressed in E. coli
Niveau de qualité
Essai
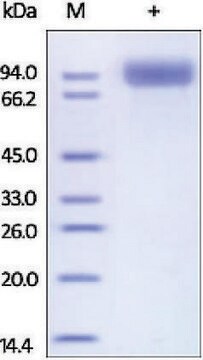
≥90% (SDS-PAGE)
Forme
solution
Activité spécifique
≥10 unit/μg protein
Poids mol.
81.6 kDa
Conditions d'expédition
dry ice
Température de stockage
−70°C
Description générale
Prolyl oligopeptidase (PO) is mapped to human chromosome 6q22. It comprises of a N-terminal β-propeller domain and C-terminal α/β hydrolase catalytic domain. PO is highly expressed in the neuronal cytoplasm and its expression increases with age.
Prolyl oligopeptidase is a cytosolic serine peptidase which cleaves peptide bonds at the C′ terminal side of prolines. It is only capable of processing peptides containing no more than 30 amino acids due to the unique β-propeller region that regulates access to the active site.
Application
Prolyl oligopeptidase has been used in a study to assess the mechanism of lithium ion action. It has also been used in a study to investigate its distribution in human tissue and body fluids.
Prolyl oligopeptidase has been used in the prolyl oligopeptidase inhibitory activity assay in lyophilized protein hydrolysate samples and Schistosoma mansoni samples.
Actions biochimiques/physiologiques
Prolyl oligopeptidase (PO) regulates physiological processes and is crucial for the generation of active hormone and peptide fragments from their precursors. It shows elevated levels in psychiatric disorders and Alzheimer′s patients. Altered levels of PO is observed in patients with bipolar disorder. High expression of PO may promote metastasis of malignant ovarian and colorectal tumors.
Définition de l'unité
One unit will hydrolyze 1.0 picomole of Ala-Pro-aminomethylcoumarin per minute at pH 7.4 at 25 °C.
Forme physique
Supplied as a solution in 45 mM Tris-HCl, pH 8.0, 124 mM NaCl, 2.4 mM KCl, 10% glycerol, 3 mM DTT and variable amounts of imidazole.
Notes préparatoires
N-terminal GST-tagged 81.6 kDa full-length protein
Code de la classe de stockage
12 - Non Combustible Liquids
Classe de danger pour l'eau (WGK)
WGK 1
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Faites votre choix parmi les versions les plus récentes :
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Prolyl oligopeptidase and bipolar disorder
Williams, Robin SB
Clinical neuroscience research, 4(3-4), 233-242 (2004)
L Polgár
Cellular and molecular life sciences : CMLS, 59(2), 349-362 (2002-03-28)
A group of serine peptidases, the prolyl oligopeptidase family, cannot hydrolyze peptides containing more than about 30 residues. This group is unrelated to the classical trypsin and subtilisin families, and includes dipeptidyl peptidase IV, acylaminoacyl peptidase and oligopeptidase B, in
Distribution of Prolyl Oligopeptidase in Human Peripheral Tissues and Body Fluids
Goossens, F., et al.
Clinical Chemistry and Laboratory Medicine, 34, 17-22 null
Prolyl oligopeptidase from the blood fluke Schistosoma mansoni: from functional analysis to anti-schistosomal inhibitors
Fajtova P, et al.
PLoS Neglected Tropical Diseases, 9(6), e0003827-e0003827 (2015)
Distribution of prolyl oligopeptidase in human peripheral tissues and in ovarian and colorectal tumors
Myohanen TT, et al.
The Journal of Histochemistry and Cytochemistry, 60(9), 706-715 (2012)
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique