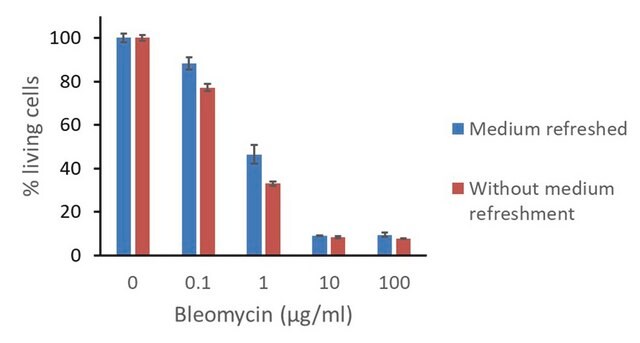
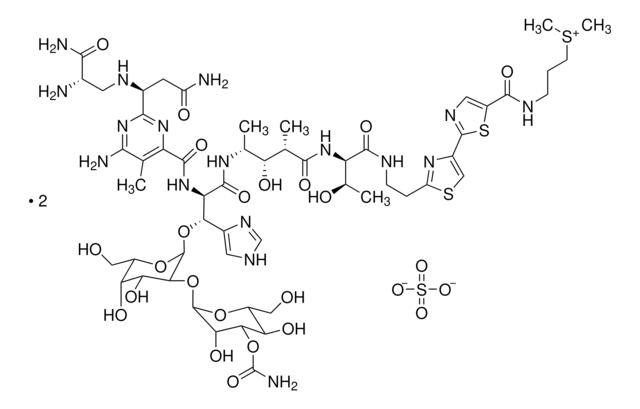
N9162
Neocarzinostatin
from Streptomyces carzinostaticus, ≥90% (SDS-PAGE), solution
Synonyme(s) :
Holoneocarzinostatin, NCS, NSC-69856, Zinostatin
About This Item
Produits recommandés
Nom du produit
Neocarzinostatin from Streptomyces carzinostaticus, ≥90% (SDS-PAGE), ~0.5 mg/mL
Niveau de qualité
Essai
≥90% (SDS-PAGE)
Forme
solution
Concentration
~0.5 mg/mL
Spectre d'activité de l'antibiotique
neoplastics
Mode d’action
DNA synthesis | interferes
Conditions d'expédition
wet ice
Température de stockage
2-8°C
Chaîne SMILES
N([C@H]1[C@H](O[C@@H]([C@@H]([C@@H]1O)O)C)O[C@H]2[C@@H](C=C5C2CC#C[C@H]6O[C@]6(C#C5)[C@@H]7OC(=O)OC7)OC(=O)c3c4c(c(cc(c4)OC)C)ccc3O)C
InChI
1S/C35H35NO12/c1-16-12-19(42-4)14-22-20(16)8-9-23(37)27(22)32(40)45-24-13-18-10-11-35(26-15-43-34(41)46-26)25(48-35)7-5-6-21(18)31(24)47-33-28(36-3)30(39)29(38)17(2)44-33/h8-9,12-14,17,21,24-26,28-31,33,36-39H,6,15H2,1-4H3/t17-,21?,24-,25-,26-,28-,29+,30-,31-,33-,35+/m1/s1
Clé InChI
BLXZMHNVKCEIJX-PNKAXBHOSA-N
Application
- as a DNA damaging agent to treat human fibroblast GM0637 cells
- as a radiomimetic antibiotic to induce DNA damage
- in combination with Vγ2Vδ2 T cells to determine the cytotoxicity in ovarian cancer cells
Actions biochimiques/physiologiques
Forme physique
Code de la classe de stockage
10 - Combustible liquids
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Équipement de protection individuelle
Eyeshields, Gloves, multi-purpose combination respirator cartridge (US)
Faites votre choix parmi les versions les plus récentes :
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique