G8415
L-Glutamic acid
98.5-100.5%, suitable for cell culture, non-animal source, meets EP testing specifications
Synonyme(s) :
(S)-2-Aminopentanedioic acid, Glu
About This Item
Produits recommandés
Nom du produit
L-Glutamic acid, from non-animal source, meets EP testing specifications, suitable for cell culture, 98.5-100.5%
Source biologique
non-animal source
Niveau de qualité
Agence
meets EP testing specifications
Essai
98.5-100.5%
Forme
powder
Technique(s)
cell culture | mammalian: suitable
Impuretés
endotoxin, tested
Couleur
white
Pf
205 °C (dec.) (lit.)
Solubilité
1 M HCl: 100 mg/mL
Densité
1.54 g/cm3 at 20 °C
Traces d'anions
chloride (Cl-): ≤200 ppm
sulfate (SO42-): ≤200 ppm
Traces de cations
As: ≤1 ppm, passes test
Fe: ≤10 ppm, passes test
NH4+: ≤200 ppm, passes test
Application(s)
pharmaceutical (small molecule)
Chaîne SMILES
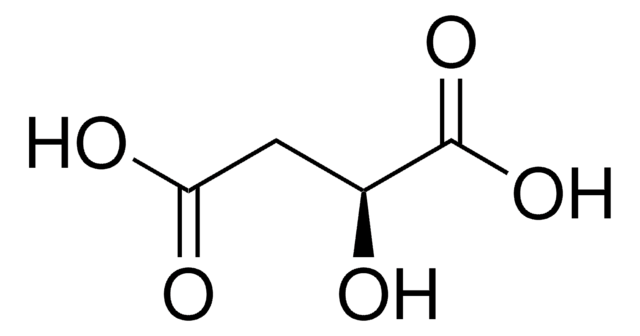
N[C@@H](CCC(O)=O)C(O)=O
InChI
1S/C5H9NO4/c6-3(5(9)10)1-2-4(7)8/h3H,1-2,6H2,(H,7,8)(H,9,10)/t3-/m0/s1
Clé InChI
WHUUTDBJXJRKMK-VKHMYHEASA-N
Informations sur le gène
human ... CCR2(1231) , GRIA1(2890) , GRIA2(2891) , GRIA4(2893) , GRIK1(2897) , GRIK2(2898) , GRIK3(2899) , GRIK5(2901) , GRIN2B(2904) , GRM2(2912) , SLC1A1(6505) , SLC1A2(6506)
rat ... Gria1(50592) , Grik1(29559) , Grik2(54257) , Grik4(24406) , Grin2a(24409) , Grm1(24414) , Grm2(24415) , Grm3(24416) , Grm4(24417) , Grm5(24418) , Grm6(24419) , Grm7(81672) , Slc1a2(29482)
Vous recherchez des produits similaires ? Visite Guide de comparaison des produits
Catégories apparentées
Application
Actions biochimiques/physiologiques
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 1
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Équipement de protection individuelle
Eyeshields, Gloves, type N95 (US)
Faites votre choix parmi les versions les plus récentes :
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
guidance
Articles
Sigma-Aldrich presents an article about how proliferatively active cells require both a source of carbon and of nitrogen for the synthesis of macromolecules. Although a large proportion of tumor cells utilize aerobic glycolysis and shunt metabolites away from mitochondrial oxidative phosphorylation, many tumor cells exhibit increased mitochondrial activity.
Chromatograms
application for HPLCNotre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique