D9568
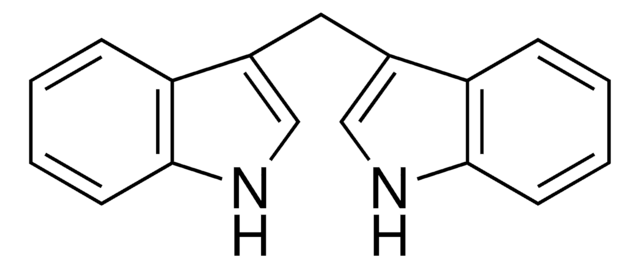
3,3′-Diindolylmethane
≥98% (HPLC)
Synonyme(s) :
DIM
About This Item
Produits recommandés
Niveau de qualité
Pureté
≥98% (HPLC)
Forme
powder
Température de stockage
2-8°C
Chaîne SMILES
C(c1c[nH]c2ccccc12)c3c[nH]c4ccccc34
InChI
1S/C17H14N2/c1-3-7-16-14(5-1)12(10-18-16)9-13-11-19-17-8-4-2-6-15(13)17/h1-8,10-11,18-19H,9H2
Clé InChI
VFTRKSBEFQDZKX-UHFFFAOYSA-N
Vous recherchez des produits similaires ? Visite Guide de comparaison des produits
Application
- 3,3′-Diindolylmethane inhibits Th17 cell differentiation via impairing IRF-7-mediated plasmacytoid dendritic cell activation in imiquimod-induced psoriasis mice.: The research indicates that 3,3′-Diindolylmethane can effectively inhibit Th17 cell differentiation, offering a potential therapeutic approach for treating psoriasis by targeting plasmacytoid dendritic cell pathways (Rasool et al., 2024).
- Protective effect of diindolylmethane-enriched dietary cabbage against doxorubicin-induced cardiotoxicity in mice.: This study highlights the cardioprotective effects of a diindolylmethane-enriched diet in mice, offering a dietary approach to mitigate the cardiotoxic effects of doxorubicin, a common chemotherapeutic agent (Natesh et al., 2024).
- Nanoformulated 3′-diindolylmethane modulates apoptosis, migration, and angiogenesis in breast cancer cells.: The investigation into nanoformulated 3′-diindolylmethane shows it significantly influences apoptosis, migration, and angiogenesis, suggesting its utility in targeted cancer therapies (Harakeh et al., 2024).
- Design, synthesis, and biological evaluation of 3,3′-diindolylmethane N-linked glycoconjugate as a leishmanial topoisomerase IB inhibitor with reduced cytotoxicity.: Research presents a synthesized glycoconjugate of 3,3′-Diindolylmethane as an effective inhibitor of leishmanial topoisomerase IB, demonstrating reduced cytotoxicity and potential as a therapeutic agent (Kour et al., 2023).
Actions biochimiques/physiologiques
Mention d'avertissement
Warning
Mentions de danger
Conseils de prudence
Classification des risques
Aquatic Chronic 4 - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Organes cibles
Respiratory system
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Équipement de protection individuelle
dust mask type N95 (US), Eyeshields, Gloves
Certificats d'analyse (COA)
Recherchez un Certificats d'analyse (COA) en saisissant le numéro de lot du produit. Les numéros de lot figurent sur l'étiquette du produit après les mots "Lot" ou "Batch".
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique