75762
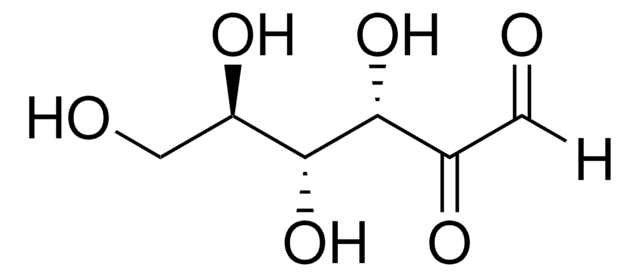
3-Deoxyglucosone
≥75% (TLC)
Synonyme(s) :
3-Deoxy-D-erythro-hexosulose
Se connecterpour consulter vos tarifs contractuels et ceux de votre entreprise/organisme
About This Item
Formule empirique (notation de Hill) :
C6H10O5
Numéro CAS:
Poids moléculaire :
162.14
Numéro MDL:
Code UNSPSC :
12352201
ID de substance PubChem :
Nomenclature NACRES :
NA.25
Produits recommandés
Niveau de qualité
Essai
≥75% (TLC)
Forme
solid
Couleur
faintly yellow to orange
Température de stockage
room temp
Chaîne SMILES
OC[C@H]1OC(O)C(=O)C[C@@H]1O
InChI
1S/C6H10O5/c7-2-5-3(8)1-4(9)6(10)11-5/h3,5-8,10H,1-2H2/t3-,5+,6?/m0/s1
Clé InChI
UHPMJDGOAZMIID-OPAZFOKUSA-N
Application
3-Deoxyglucosone is used as a reference for the analysis and detection of glucose degradation products, glycating agents, generated by processes such as heat sterilization.
Autres remarques
To gain a comprehensive understanding of our extensive range of Monosaccharides for your research, we encourage you to visit our Carbohydrates Category page.
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Équipement de protection individuelle
Eyeshields, Gloves, type N95 (US)
Faites votre choix parmi les versions les plus récentes :
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Michael Hellwig et al.
Journal of agricultural and food chemistry, 58(19), 10752-10760 (2010-09-09)
1,2-Dicarbonyl compounds are formed in food during Maillard and caramelization reactions. 3-Deoxy-D-threo-hexos-2-ulose (3-deoxygalactosone, 3-DGal) and galactosone, two 1,2-dicarbonyl compounds originating from the degradation of galactose, were synthesized and converted to the respective quinoxalines, which were characterized by NMR spectroscopy. Analytical
He Li et al.
Journal of agricultural and food chemistry, 67(32), 9050-9059 (2019-07-25)
The control of 2,3-dihydro-3,5-dihydroxy-6-methyl-4(H)-pyran-4-one (DDMP) formation in the Maillard reaction is important to improve the thermally treated food quality as a result of its intense bitterness and potential toxicity. In this work, phenolic acids, such as gallic, protocatechuic, caffeic, and
Danielle T Loughlin et al.
Wound repair and regeneration : official publication of the Wound Healing Society [and] the European Tissue Repair Society, 17(5), 739-749 (2009-09-23)
The interaction of fibroblasts with the extracellular matrix is critical for wound healing. Advanced glycation end products (AGEs) occur through nonenzymatic glycation of long-lived proteins such as collagens. One precursor to these modifications, 3-deoxyglucosone (3DG), is elevated in patients with
Alina Shapira et al.
Molecular nutrition & food research, 51(4), 473-478 (2007-03-29)
Peritoneal dialysis (PD) is commonly performed by using preprepared dialysis solutions containing glucose, which are thermally treated to achieve commercial sterilization. A series of glucose degradation products (GDPs) are being formed, which react with the tissue during the dialysis procedure
Martin Erixon et al.
Peritoneal dialysis international : journal of the International Society for Peritoneal Dialysis, 28(3), 277-282 (2008-05-14)
Glucose degradation products (GDPs) are important in the outcome of peritoneal dialysis (PD) treatment. 3,4-dideoxyglucosone-3-ene (3,4-DGE) is the most cytotoxic GDP found in conventionally manufactured fluids and may, in addition, be recruited from 3-deoxyglucosone (3-DG). It is not known what
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique