10810
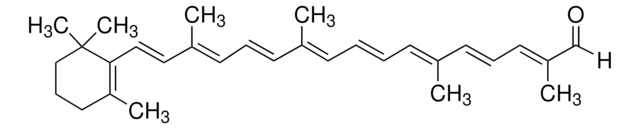
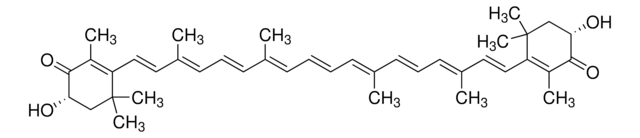
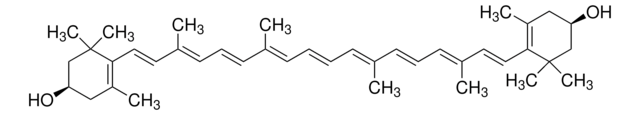
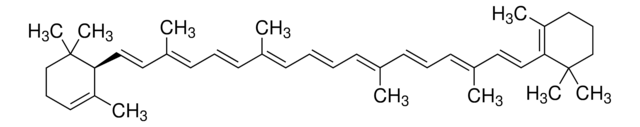
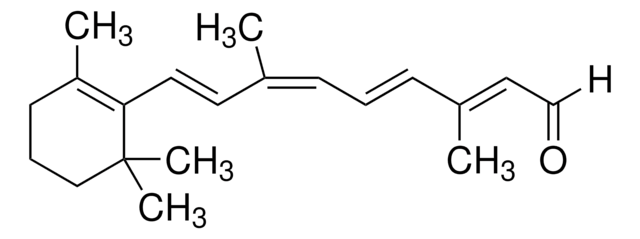
trans-β-Apo-8′-carotenal
≥96.0% (UV)
Synonyme(s) :
Apocarotenal
About This Item
Produits recommandés
Source biologique
synthetic
Niveau de qualité
Essai
≥96.0% (UV)
Forme
powder
Perte
≤0.5% loss on drying, 20 °C (HV)
Pf
137-141 °C
Solubilité
chloroform: 1 mg/mL, clear to very faintly turbid, intense red-orange
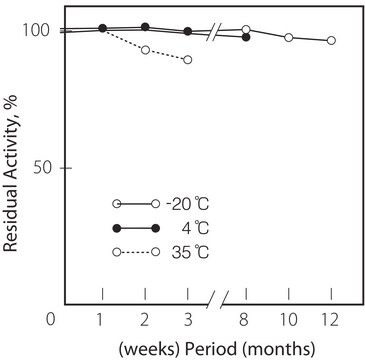
Température de stockage
−20°C
Chaîne SMILES
[H]C(=O)\C(C)=C\C=C\C(C)=C\C=C\C=C(C)\C=C\C=C(C)\C=C\C1=C(C)CCCC1(C)C
InChI
1S/C30H40O/c1-24(13-8-9-14-25(2)16-11-18-27(4)23-31)15-10-17-26(3)20-21-29-28(5)19-12-22-30(29,6)7/h8-11,13-18,20-21,23H,12,19,22H2,1-7H3/b9-8+,15-10+,16-11+,21-20+,24-13+,25-14+,26-17+,27-18+
Clé InChI
DFMMVLFMMAQXHZ-DOKBYWHISA-N
Vous recherchez des produits similaires ? Visite Guide de comparaison des produits
Application
- Tackling the challenge of selective analytical clean-up of complex natural extracts: the curious case of chlorophyll removal: This study outlines a methodology for the selective analytical clean-up of complex natural extracts, which can be applied to enhance the purity and stability of trans-β-Apo-8′-carotenal, particularly useful in the context of life science manufacturing and research and development. This approach is crucial for maintaining the integrity of bioactive compounds during synthesis and storage (Bijttebier et al., 2014).
Actions biochimiques/physiologiques
Autres remarques
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 2
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Équipement de protection individuelle
Eyeshields, Gloves, type N95 (US)
Faites votre choix parmi les versions les plus récentes :
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique