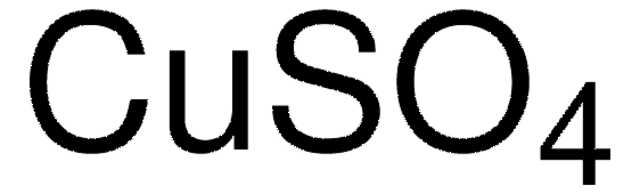209198
Copper(II) sulfate pentahydrate
ACS reagent, ≥98.0%
Synonyme(s) :
Cupric sulfate pentahydrate, Sulfuric acid, copper(II) salt (1:1) pentahydrate
About This Item
Produits recommandés
Qualité
ACS reagent
Niveau de qualité
Pression de vapeur
7.3 mmHg ( 25 °C)
Essai
≥98.0%
98.0-102.0% (ACS specification)
Forme
crystals
Capacité de réaction
reaction type: click chemistry
Pertinence de la réaction
reagent type: catalyst
core: copper
pH
3.5-4.5 (20 °C, 50 g/L)
Pf
110 °C (dec.) (lit.)
Traces d'anions
chloride (Cl-): ≤0.001%
Traces de cations
Ca: ≤0.005%
Fe: ≤0.003%
K: ≤0.01%
Na: ≤0.02%
Ni: ≤0.005%
Chaîne SMILES
O.O.O.O.O.[Cu++].[O-]S([O-])(=O)=O
InChI
1S/Cu.H2O4S.5H2O/c;1-5(2,3)4;;;;;/h;(H2,1,2,3,4);5*1H2/q+2;;;;;;/p-2
Clé InChI
JZCCFEFSEZPSOG-UHFFFAOYSA-L
Vous recherchez des produits similaires ? Visite Guide de comparaison des produits
Description générale
Application
- In acetylation of alcohols and phenols in the presence of acetic anhydride.
- To synthesize various ynamides via intramolecular coupling of amides with alkynyl bromides.
It can also be used as a template in the synthesis of copper(II) ion-imprinted polymers using vinylpyridine and methacrylic acid as functional monomers. This polymer is used to quantify Cu2+ ion by using flame atomic absorption spectroscopy.
Caractéristiques et avantages
Mention d'avertissement
Danger
Mentions de danger
Conseils de prudence
Classification des risques
Acute Tox. 4 Oral - Aquatic Acute 1 - Aquatic Chronic 1 - Eye Dam. 1
Code de la classe de stockage
13 - Non Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Faites votre choix parmi les versions les plus récentes :
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique