PHR1854
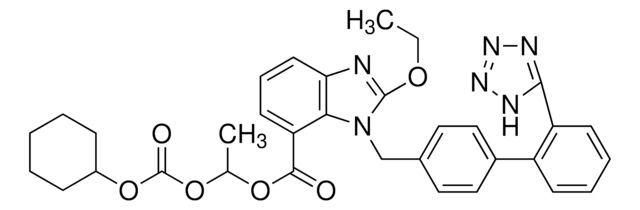
Candesartan Cilexetil
Pharmaceutical Secondary Standard; Certified Reference Material
Synonyme(s) :
Candesartan cilexetil, 2-ethoxy-1-[[2′-(2H-tetrazol-5-yl)[1,1′-biphenyl]-4-yl]methyl]-1H-Benzimidazole-7-carboxylic acid 1-[[(cyclohexyloxy)carbonyl]oxy]ethyl ester, TCV 116, TCY 116
About This Item
Produits recommandés
Qualité
certified reference material
pharmaceutical secondary standard
Niveau de qualité
Agence
traceable to Ph. Eur. Y0001388
traceable to USP 1087803
Famille d'API
candesartan
Forme
powder
Conditionnement
pkg of 200 mg
Application(s)
pharmaceutical
Chaîne SMILES
CCOc1nc2cccc(C(=O)OC(C)OC(=O)OC3CCCCC3)c2n1Cc4ccc(cc4)-c5ccccc5-c6nnn[nH]6
InChI
1S/C33H34N6O6/c1-3-42-32-34-28-15-9-14-27(31(40)43-21(2)44-33(41)45-24-10-5-4-6-11-24)29(28)39(32)20-22-16-18-23(19-17-22)25-12-7-8-13-26(25)30-35-37-38-36-30/h7-9,12-19,21,24H,3-6,10-11,20H2,1-2H3,(H,35,36,37,38)
Clé InChI
GHOSNRCGJFBJIB-UHFFFAOYSA-N
Informations sur le gène
human ... AGTR1(185)
Vous recherchez des produits similaires ? Visite Guide de comparaison des produits
Description générale
Pharmaceutical secondary standards for application in quality control provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to the preparation of in-house working standards.
Candesartan cilexetil is an angiotensin II receptor antagonist used as a prodrug in the treatment of hypertension.
Application
- Determination of candesartan cilexetil in tablet formulations by a UV/fluorescence spectrophotometric method
- Study of the release of candesartan cilexetil in tablet form by reversed-phase high-performance liquid chromatography (RP-HPLC)
- Simultaneous estimation of candesartan cilexetil and hydrochlorothiazide in pharmaceutical preparations using liquid chromatography in combination with photodiode array detector (DAD) and evaporative light scattering detector (ELSD)
- Spectroflourimetric determination of four angiotensin II receptor antagonists (AIIRA’s) in their pure form as well as pharmaceutical formulations
Actions biochimiques/physiologiques
Remarque sur l'analyse
Note de bas de page
Produits recommandés
Produit(s) apparenté(s)
Mention d'avertissement
Danger
Mentions de danger
Conseils de prudence
Classification des risques
Repr. 1B - STOT RE 2 Oral
Organes cibles
Kidney,Blood
Code de la classe de stockage
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Faites votre choix parmi les versions les plus récentes :
Certificats d'analyse (COA)
Désolés, nous n'avons pas de COA pour ce produit disponible en ligne pour le moment.
Si vous avez besoin d'assistance, veuillez contacter Service Clients
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique