PHR1752
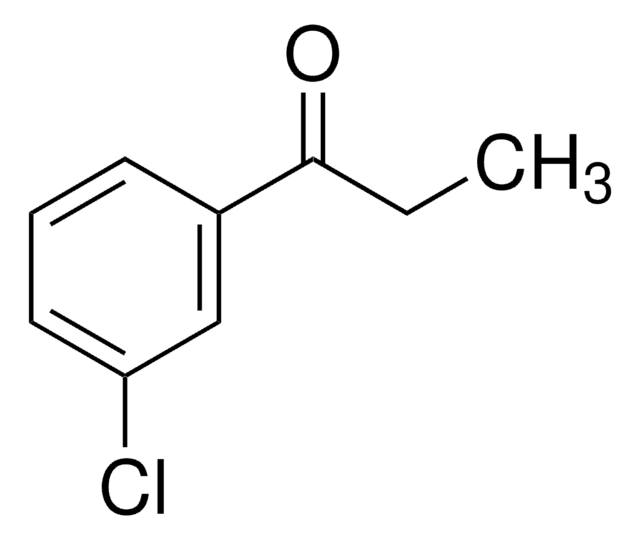
Bupropion Hydrochloride Related Compound A
Pharmaceutical Secondary Standard; Certified Reference Material
Synonyme(s) :
2-(tert-Butylamino)-4′-chloropropiophenone hydrochloride, 2-(tert-butylamino)-3’-bromopiophenone hydrochloride
About This Item
Produits recommandés
Qualité
certified reference material
pharmaceutical secondary standard
Niveau de qualité
Agence
traceable to USP 1078744
Famille d'API
bupropion
CofA (certificat d'analyse)
current certificate can be downloaded
Conditionnement
pkg of 30 mg
Technique(s)
HPLC: suitable
gas chromatography (GC): suitable
Application(s)
pharmaceutical (small molecule)
Format
neat
Température de stockage
2-8°C
Vous recherchez des produits similaires ? Visite Guide de comparaison des produits
Description générale
Bupropion hydrochloride (BUP) and its derivatives belong to a chemical class of aminoketones and are known for their antidepressant abilities. BUP selectively inhibits the neuronal reabsorption of catecholamines (noradrenalin and dopamine), has minimal effect on the recapture of indolamines (serotonin) and no inhibitory effect on monoamine oxidase. It is used as the non-nicotine pharmacological therapy for combating smoking in controlled release form.
Bupropion hydrochloride (BUP) may be used as a pharmaceutical reference standard for the determination of the analyte in pharmaceutical formulations by spectrophotometric and chromatographic techniques.
Application
Remarque sur l'analyse
Autres remarques
Note de bas de page
Produits recommandés
Produit(s) apparenté(s)
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Faites votre choix parmi les versions les plus récentes :
Certificats d'analyse (COA)
Vous ne trouvez pas la bonne version ?
Si vous avez besoin d'une version particulière, vous pouvez rechercher un certificat spécifique par le numéro de lot.
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique


![4-[(1RS)-2-(tert-Butylamino)-1-hydroxyethyl]-2-ethylphenol hydrochloride certified reference material, pharmaceutical secondary standard](/deepweb/assets/sigmaaldrich/product/images/399/698/c88a0004-aea6-4f13-9d6a-09d648ff9eb0/640/c88a0004-aea6-4f13-9d6a-09d648ff9eb0.jpg)