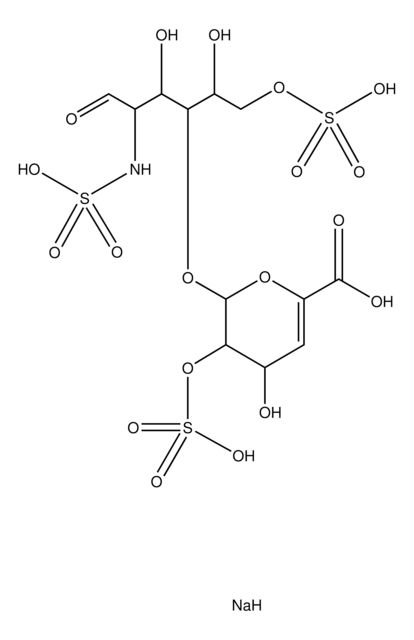
H0200000
Heparin sodium
BRP, European Pharmacopoeia (EP) Reference Standard
About This Item
Produits recommandés
Qualité
pharmaceutical primary standard
Famille d'API
heparin
Fabricant/nom de marque
EDQM
Application(s)
pharmaceutical (small molecule)
Format
neat
Vous recherchez des produits similaires ? Visite Guide de comparaison des produits
Description générale
For further information and support please go to the website of the issuing Pharmacopoeia.
Application
Established for use in the:
- Assay of unfractionated heparin according to the Ph. Eur. General text 2.7.5. with assigned contents of 985 IU/mL for anti-IIa and 995 IU/mL for anti-Xa activities
- Test for heparin in human antithrombin III according to the Ph. Eur. Monograph 0878 with an assigned content of 1035 IU/mL for sheep plasma clotting assay
- Assay of protamine sulfate according to the prescriptions of the Ph. Eur. Monograph 0569
Conditionnement
Autres remarques
Produit(s) apparenté(s)
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 2
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Faites votre choix parmi les versions les plus récentes :
Certificats d'analyse (COA)
Désolés, nous n'avons pas de COA pour ce produit disponible en ligne pour le moment.
Si vous avez besoin d'assistance, veuillez contacter Service Clients
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique