38370
DIC
≥98.0% (GC), for peptide synthesis
Synonyme(s) :
N,N′-Diisopropylcarbodiimide
About This Item
Produits recommandés
product name
DIC, purum, ≥98.0% (GC)
Qualité
purum
Niveau de qualité
Pureté
≥98.0% (GC)
Forme
liquid
Capacité de réaction
reaction type: Coupling Reactions
Indice de réfraction
n20/D 1.433 (lit.)
Point d'ébullition
145-148 °C (lit.)
Densité
0.815 g/mL at 20 °C (lit.)
0.815 g/mL at 20 °C
Application(s)
peptide synthesis
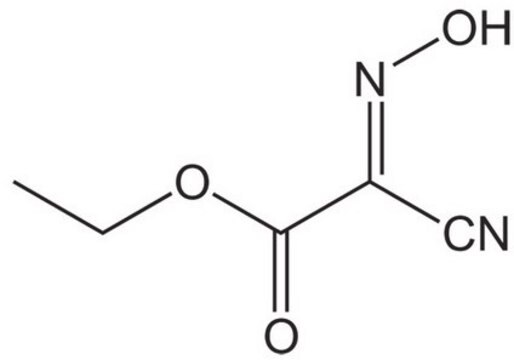
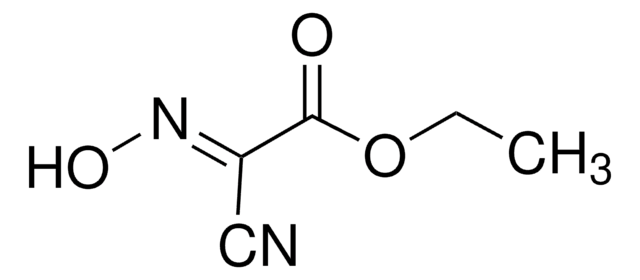
Chaîne SMILES
CC(C)N=C=NC(C)C
InChI
1S/C7H14N2/c1-6(2)8-5-9-7(3)4/h6-7H,1-4H3
Clé InChI
BDNKZNFMNDZQMI-UHFFFAOYSA-N
Informations sur le gène
human ... EPHX2(2053)
mouse ... Ephx2(13850)
Vous recherchez des produits similaires ? Visite Guide de comparaison des produits
Catégories apparentées
Description générale
Application
- To synthesize lanthanide (Ln) guanidinate complexes via insertion of carbodiimide into the Ln-N bond of lanthanocene secondary amido complexes.
- To facilitate the cyclization of N-(β-Hydroxy)amides to form 2-oxazolines.
- To synthesize 1-isopropyl-2-alkoxycarbonyl-3-isopropyliminio-aziridine by reacting with alkyl diazoacetates in the presence of transition metal salts.
- A coupling reagent for the synthesis of various esters and amides by treating carboxylic acids with phenols and amines respectively.
- A reagent for the conversion of alcohols to aldehydes or ketones in the presence of DMSO via modified Moffatt-type oxidation reaction.
- A reagent to facilitates the preparation of alkyl halides from corresponding alcohols via the formation of o-alkylisourea.
Produit(s) apparenté(s)
Mention d'avertissement
Danger
Mentions de danger
Classification des risques
Acute Tox. 1 Inhalation - Eye Dam. 1 - Flam. Liq. 3 - Resp. Sens. 1 - Skin Sens. 1
Code de la classe de stockage
3 - Flammable liquids
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
91.4 °F
Point d'éclair (°C)
33 °C
Équipement de protection individuelle
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
Faites votre choix parmi les versions les plus récentes :
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Articles
In principle, the seemingly simple formation of a peptide bond can be accomplished using all the procedures available in organic chemistry for the synthesis of carboxylic acid amides. However, due to the presence of various functional groups in natural and unnatural amino acids and particularly the requirement for full retention of chiral integrity, the coupling of amino acids and peptides under mild conditions can be challenging. A plethora of coupling reagents has been developed superseding each other in efficiency and suitability for specific applications (e.g., solid-phase peptide synthesis or fragment condensation).
In principle, the seemingly simple formation of a peptide bond can be accomplished using all the procedures available in organic chemistry for the synthesis of carboxylic acid amides. However, due to the presence of various functional groups in natural and unnatural amino acids and particularly the requirement for full retention of chiral integrity, the coupling of amino acids and peptides under mild conditions can be challenging. A plethora of coupling reagents has been developed superseding each other in efficiency and suitability for specific applications (e.g., solid-phase peptide synthesis or fragment condensation).
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique