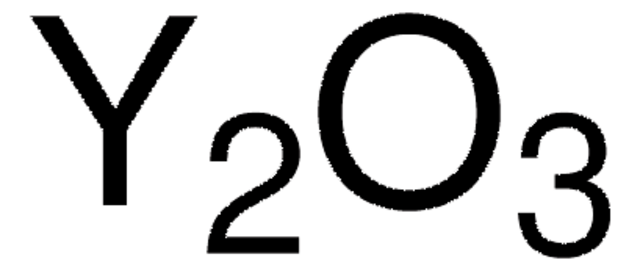L4000
Lanthanum(III) oxide
≥99.9%
Synonyme(s) :
Lanthana, Lanthanum sesquioxide, Lanthanum trioxide
About This Item
Produits recommandés
Pureté
≥99.9%
Forme
powder
Pertinence de la réaction
reagent type: catalyst
core: lanthanum
Densité
6.51 g/mL at 25 °C (lit.)
Application(s)
battery manufacturing
Chaîne SMILES
O=[La]O[La]=O
InChI
1S/2La.3O
Clé InChI
KTUFCUMIWABKDW-UHFFFAOYSA-N
Vous recherchez des produits similaires ? Visite Guide de comparaison des produits
Description générale
Application
It can be used to prepare thermal-barrier coatings with a high thermal expansion coefficient and low thermal conductivity.
It can also be used as a recyclable catalytic system for the synthesis of diphenyl sulfides and selenides.
Caractéristiques et avantages
- High refractive index
- Thermal stability
- Hardness
- High dielectric constant
Code de la classe de stockage
13 - Non Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Équipement de protection individuelle
dust mask type N95 (US), Eyeshields, Gloves
Faites votre choix parmi les versions les plus récentes :
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Articles
Innovation in dental restorative materials is driven by the need for biocompatible and natural-appearing restoration alternatives. Conventional dental materials like amalgam and composite resins have inherent disadvantages.
Rechargeable solid-state batteries are becoming increasingly important due to wide-spread use in computers, portable electronics, and vehicular applications.
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique