B103500
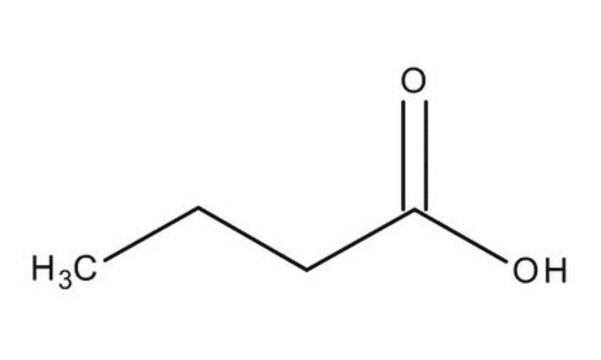
Acide butyrique
≥99%
Synonyme(s) :
1-Propanecarboxylic acid, Ethylacetic acid, Propylformic acid, n-Butanoic acid, n-Butyric acid
About This Item
Produits recommandés
Densité de vapeur
3.04 (vs air)
Niveau de qualité
Pression de vapeur
0.43 mmHg ( 20 °C)
Essai
≥99%
Forme
liquid
Température d'inflammation spontanée
824 °F
Limite d'explosivité
10 %
Technique(s)
HPLC: suitable
Indice de réfraction
n20/D 1.398 (lit.)
pH
2 (25 °C, 10 g/L)
3 (20 °C, 10 g/L)
pb
162 °C (lit.)
Pf
−6-−3 °C (lit.)
Densité
0.964 g/mL at 25 °C (lit.)
Chaîne SMILES
CCCC(O)=O
InChI
1S/C4H8O2/c1-2-3-4(5)6/h2-3H2,1H3,(H,5,6)
Clé InChI
FERIUCNNQQJTOY-UHFFFAOYSA-N
Informations sur le gène
human ... HDAC1(3065)
Vous recherchez des produits similaires ? Visite Guide de comparaison des produits
Catégories apparentées
Application
Mention d'avertissement
Danger
Mentions de danger
Classification des risques
Acute Tox. 4 Oral - Eye Dam. 1 - Skin Corr. 1B
Code de la classe de stockage
8A - Combustible, corrosive hazardous materials
Classe de danger pour l'eau (WGK)
WGK 1
Point d'éclair (°F)
161.6 °F - closed cup
Point d'éclair (°C)
72 °C - closed cup
Équipement de protection individuelle
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
Faites votre choix parmi les versions les plus récentes :
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Articles
Separation of Propionic acid; Acetic acid; Heptanoic acid; Isobutyric acid; Valeric acid; Isocaproic acid; Butyric acid; Isovaleric acid
Separation of Methyl oleate; Caprylic acid; Heptanoic acid; Methyl decanoate; Methyl dodecanoate; Myristic acid; Methyl palmitate; Methyl palmitoleate; Methyl stearate; Methyl linoleate; Methyl linolenate; Acetic acid; Arachidic acid; Behenic acid; Propionic acid; Isobutyric acid; Valeric acid; Isovaleric acid; Isocaproic acid; Butyric acid
probiotics-and-human
Today, diverse studies report the benefits of probiotics, such as inhibitory effects on pathogens, aid in the management or prevention of chronic intestinal inflammatory diseases or atopic syndromes, and support to the immune system. Potential beneficial applications abound, researchers continue to evaluate the effictiveness and clarify the mechanisms of action of probiotics.
Protocoles
In this study, SPME was used for the analysis of free fatty acids in Parmesan cheese using a 65 μm Carbowax/divinylbenzene (DVB) SPME fiber. Headspace extraction of the cheese sample was conducted at 65 °C for 15 minutes and analyzed by GC with FID detection. SPME is ideal for analyzing the volatiles associated with solid food samples. The phase chemistry of the Nukol GC column provides excellent peak shape of acidic compounds.
Separation of Acetone; Acetic acid; Propionic acid; Ethyl butyrate; Ethanol; Isoamyl acetate; Isobutyric acid; 3-Methyl-2-butanol; Methyl acetate; 1-Propanol; Acetal, ≥98%, FG; 2-Methyl-1-pentanol; Butyl acetate; Ethyl propionate; 3-Pentanol; 2-Pentanol, 98%; Ethyl isobutyrate; Isobutyl acetate; Acetaldehyde; Furfural; Butyric acid; Methanol; Ethyl acetate
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique