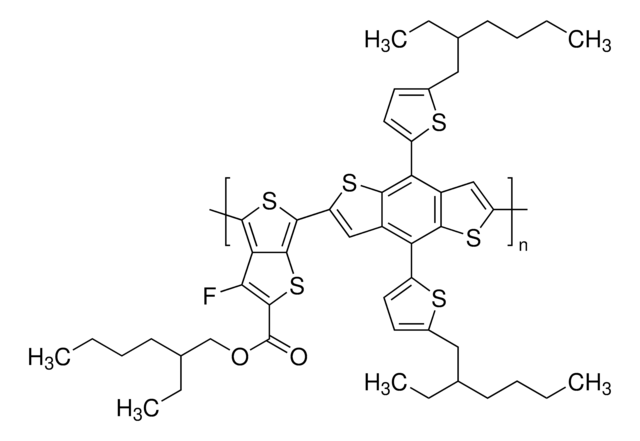
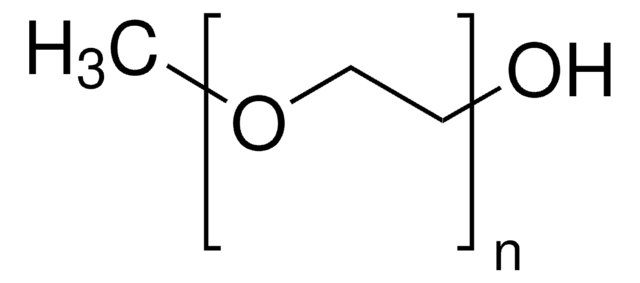
901635
Poly(ethylene glycol) bis(2-pyridyl KAT)
PEG average Mn 10,000
Synonyme(s) :
KAT PEG, bis KAT PEG, di KAT PEG
Se connecterpour consulter vos tarifs contractuels et ceux de votre entreprise/organisme
About This Item
Formule linéaire :
(C6H3BF3KNO)O[CH2CH2O]nCH2CH2O(C6H3BF3KNO)
Code UNSPSC :
12352005
Nomenclature NACRES :
NA.23
Produits recommandés
Forme
powder or solid
Poids mol.
PEG average Mn 10,000
PEG ~10,000 Da
Couleur
off-white to pale yellow
Température de stockage
2-8°C
Description générale
Poly(ethylene glycol) bis(2-pyridyl KAT) is a homobifunctional PEG featuring terminal potassium acyltrifluoroborate reactive groups for facile, rapid functionalization. Potassium acyltrifluoroborates (KATs) are stable functional groups that undergo rapid amide-forming ligations with hydroxylamines in aqueous media, in the presence of unprotected functional groups. In addition to its compatibility, these reactions proceed relatively quickly, lending to their use with sensitive biological reagents. This conjugation reaction offers a new approach to the synthesis of complex molecules without the complication of side reactions, such protein-polymer conjugates. KATs also undergo amide or imide-forming ligations in acidic conditions when reacted with primary amines or amides, respectively, as an alternative to classical acylation chemistry. Poly(ethylene glycol) bis(2-pyridyl KAT)s have been recently used in the rapid PEGylation and dimerization of expressed, folded protiens in near equimolar conditions, demonstrating the potential for these materials in a wide variety of drug delivery applications.
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Faites votre choix parmi les versions les plus récentes :
Certificats d'analyse (COA)
Lot/Batch Number
Désolés, nous n'avons pas de COA pour ce produit disponible en ligne pour le moment.
Si vous avez besoin d'assistance, veuillez contacter Service Clients
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
PEGylation and Dimerization of Expressed Proteins under Near Equimolar Conditions with Potassium 2-Pyridyl Acyltrifluoroborates.
White CJ, et al.
ACS central science (2017)
Potassium Acyltrifluoroborate (KAT) Ligations are Orthogonal to Thiol-Michael and SPAAC Reactions: Covalent Dual Immobilization of Proteins onto Synthetic PEG Hydrogels.
Mazunin D, et al.
Helvetica Chimica Acta, 100(2) (2017)
Critical evaluation and rate constants of chemoselective ligation reactions for stoichiometric conjugations in water.
Saito, et al.
ACS Chemical Biology, 10, 1026-1033 (2015)
Alberto Osuna Gálvez et al.
Journal of the American Chemical Society, 139(5), 1826-1829 (2017-01-25)
Current methods for constructing amide bonds join amines and carboxylic acids by dehydrative couplings-processes that usually require organic solvents, expensive and often dangerous coupling reagents, and masking other functional groups. Here we describe an amide formation using primary amines and
Fumito Saito et al.
ACS chemical biology, 10(4), 1026-1033 (2015-01-13)
Chemoselective ligation reactions have contributed immensely to the development of organic synthesis and chemical biology. However, the ligation of stoichiometric amounts of large molecules for applications such as protein-protein conjugates is still challenging. Conjugation reactions need to be fast enough
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique