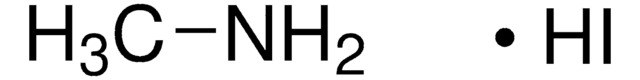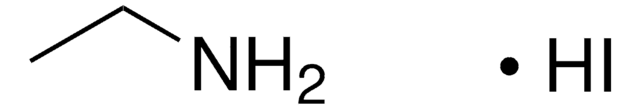793493
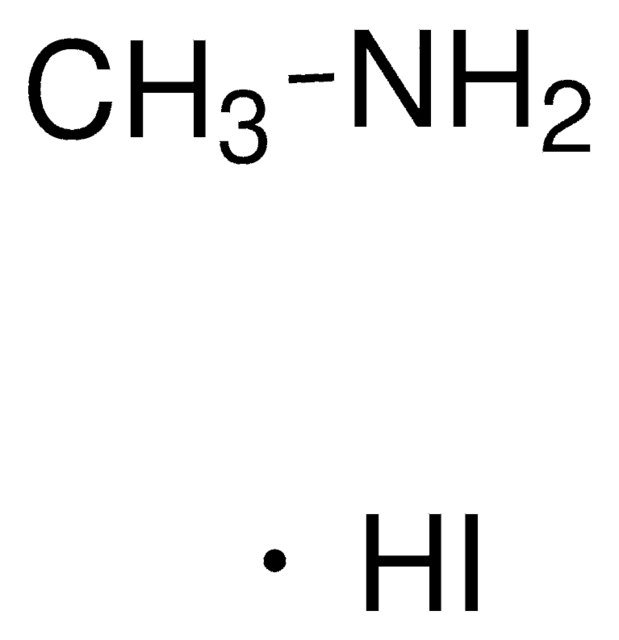
Methylammonium iodide
98%
Synonyme(s) :
Methanamine hydriodide
About This Item
Produits recommandés
Niveau de qualité
Pureté
98%
Forme
powder
Caractéristiques du produit alternatif plus écologique
Design for Energy Efficiency
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
Pf
270-280 °C
Autre catégorie plus écologique
Chaîne SMILES
CN.I
InChI
1S/CH5N.HI/c1-2;/h2H2,1H3;1H
Clé InChI
LLWRXQXPJMPHLR-UHFFFAOYSA-N
Vous recherchez des produits similaires ? Visite Guide de comparaison des produits
Application
Autres remarques
Mention d'avertissement
Warning
Mentions de danger
Conseils de prudence
Classification des risques
Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Organes cibles
Respiratory system
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Faites votre choix parmi les versions les plus récentes :
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Articles
The past several decades have seen major advancements in the synthesis of metal nanomaterials. Most recently, controlled synthesis has become versatile enough to regulate the exact number of atoms and ligands of very small metal nanoparticles, referred to as “clusters”.
The past several decades have seen major advancements in the synthesis of metal nanomaterials. Most recently, controlled synthesis has become versatile enough to regulate the exact number of atoms and ligands of very small metal nanoparticles, referred to as “clusters”.
Dr. Perini and Professor Correa-Baena discuss the latest research and effort to obtain higher performance and stability of perovskite materials.
Next generation solar cells have the potential to achieve conversion efficiencies beyond the Shockley-Queisser (S-Q) limit while also significantly lowering production costs.
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique