722987
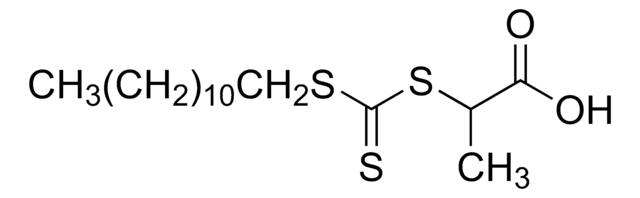
2-Cyano-2-propyl benzodithioate
>97% (HPLC)
Synonyme(s) :
2-Cyanopropan-2-yl benzodithioate
About This Item
Produits recommandés
Niveau de qualité
Essai
>97% (HPLC)
Forme
solid or liquid
Indice de réfraction
n20/D 1.621
Pf
28-31 °C
Densité
1.146 g/mL at 25 °C
Température de stockage
2-8°C
Chaîne SMILES
CC(C)(SC(=S)c1ccccc1)C#N
InChI
1S/C11H11NS2/c1-11(2,8-12)14-10(13)9-6-4-3-5-7-9/h3-7H,1-2H3
Clé InChI
IDSLBLWCPSAZBL-UHFFFAOYSA-N
Catégories apparentées
Description générale
Application
Mention d'avertissement
Warning
Mentions de danger
Conseils de prudence
Classification des risques
Skin Sens. 1
Code de la classe de stockage
10 - Combustible liquids
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
>230.0 °F - closed cup
Point d'éclair (°C)
> 110 °C - closed cup
Faites votre choix parmi les versions les plus récentes :
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Articles
A series of polymerization were carried out using RAFT agents and monomers yielding well-defined polymers with narrow molecular weight distributions. The process allows radical-initiated growing polymer chains to degeneratively transfer reactivity from one to another through the use of key functional groups (dithioesters, trithiocarbonates, xanthates and dithiocarbamates). RAFT agents help to minimize out-of-control growth and prevent unwanted termination events from occurring, effectively controlling polymer properties like molecular weight and polydispersity. RAFT agents are commercially available. RAFT does not use any cytotoxic heavy metal components (unlike ATRP).
Over the past two decades, the rapid advance of controlled living polymerization (CLP) techniques.
The modification of biomacromolecules, such as peptides and proteins, through the attachment of synthetic polymers has led to a new family of highly advanced biomaterials with enhanced properties.
We presents an article about a micro review of reversible addition/fragmentation chain transfer (RAFT) polymerization. RAFT (Reversible Addition/Fragmentation Chain Transfer) polymerization is a reversible deactivation radical polymerization (RDRP) and one of the more versatile methods for providing living characteristics to radical polymerization.
Protocoles
RAFT (Reversible Addition-Fragmentation chain Transfer) is a form of living radical polymerization involving conventional free radical polymerization of a substituted monomer in the presence of a suitable chain transfer (RAFT) reagent.
We presents an article featuring procedures that describe polymerization of methyl methacrylate and vinyl acetate homopolymers and a block copolymer as performed by researchers at CSIRO.
Sigma-Aldrich presents an article about RAFT, or Reversible Addition/Fragmentation Chain Transfer, which is a form of living radical polymerization.
Global Trade Item Number
| Référence | GTIN |
|---|---|
| 722987-1G | 4061837817229 |
| 722987-5G | 4061832860961 |
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique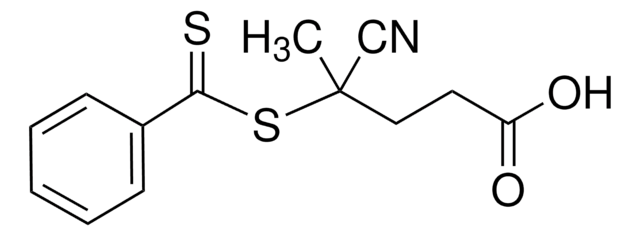
![4-Cyano-4-[(dodecylsulfanylthiocarbonyl)sulfanyl]pentanoic acid 97% (HPLC)](/deepweb/assets/sigmaaldrich/product/structures/204/925/30ae6ca0-5b0b-4963-a061-7e5e3d1a85af/640/30ae6ca0-5b0b-4963-a061-7e5e3d1a85af.png)
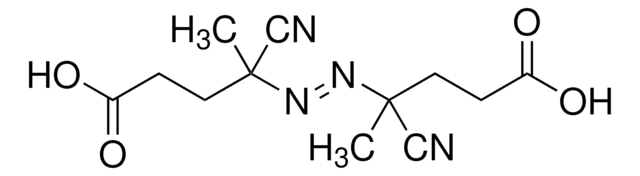
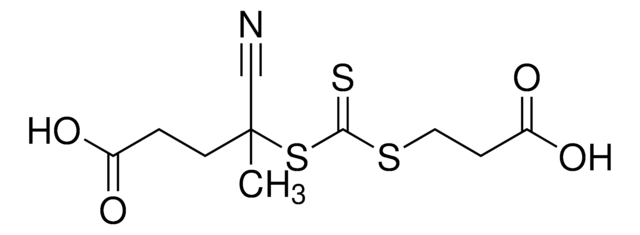
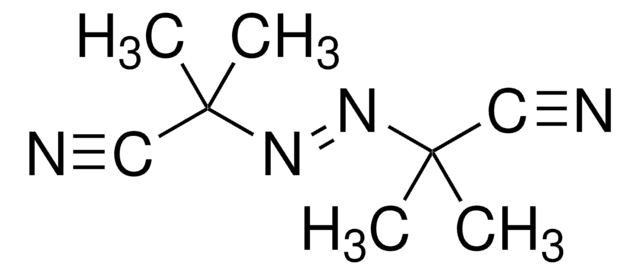
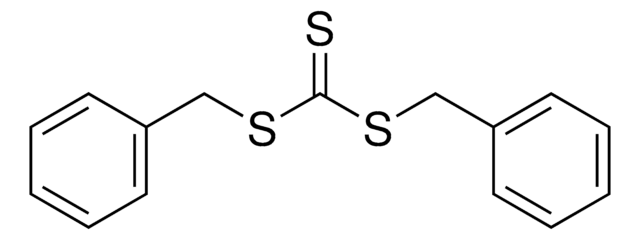
![4-Cyano-4-[(dodecylsulfanylthiocarbonyl)sulfanyl]pentanol](/deepweb/assets/sigmaaldrich/product/structures/839/520/64c23004-f340-460f-a379-8670a35d0433/640/64c23004-f340-460f-a379-8670a35d0433.png)