469971
L-Tryptophanol
97%
Se connecterpour consulter vos tarifs contractuels et ceux de votre entreprise/organisme
About This Item
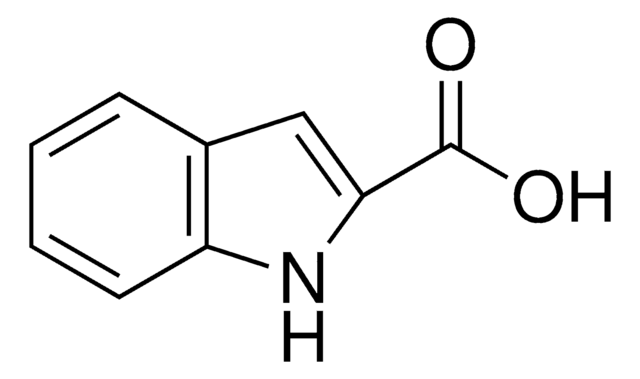
Formule empirique (notation de Hill):
C11H14N2O
Numéro CAS:
Poids moléculaire :
190.24
Numéro MDL:
Code UNSPSC :
12352209
ID de substance PubChem :
Nomenclature NACRES :
NA.22
Produits recommandés
Niveau de qualité
Pureté
97%
Activité optique
[α]20/D −20.5°, c = 1 in methanol
Capacité de réaction
reaction type: solution phase peptide synthesis
Pf
73-77 °C (lit.)
Application(s)
peptide synthesis
Chaîne SMILES
N[C@H](CO)Cc1c[nH]c2ccccc12
InChI
1S/C11H14N2O/c12-9(7-14)5-8-6-13-11-4-2-1-3-10(8)11/h1-4,6,9,13-14H,5,7,12H2/t9-/m0/s1
Clé InChI
UDQCRUSSQAXPJY-VIFPVBQESA-N
Faites votre choix parmi les versions les plus récentes :
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Theodoros Eleftheriadis et al.
International journal of molecular medicine, 42(1), 557-568 (2018-04-26)
It is generally hypothesized in the literature that indoleamine 2,3‑dioxygenase (IDO), by degrading L‑tryptophan along the kynurenine pathway, suppresses CD4+ T‑cell function by inducing apoptosis, inhibiting proliferation and promoting differentiation towards a regulatory phenotype. These effects are either accompanied or
G M Beck et al.
Chirality, 8(7), 503-510 (1996-01-01)
Lambda-carrageenan, a linear high molecular weight sulfated polysaccharide, has been employed as a chiral selector in capillary electrophoresis for the separation of enantiomers of weakly basic pharmaceutical compounds. The racemic compounds that were enantioresolved included propranolol, pindolol, tryptophanol, laudanosine and
Mercedes Amat et al.
The Journal of organic chemistry, 74(3), 1205-1211 (2008-12-17)
The enantioselective construction of the 3-ethylindolo[2,3-a]quinolizidine moiety present in numerous indole alkaloids is reported, the key steps being a stereoselective cyclocondensation of (S)-tryptophanol with an appropriate racemic delta-oxoester and a regio- and stereoselective cyclization of the resulting oxazolopiperidones on the
Ashley A Reinke et al.
Chembiochem : a European journal of chemical biology, 11(13), 1889-1895 (2010-08-03)
In Alzheimer's disease (AD) and other neurodegenerative disorders, proteins accumulate into ordered aggregates, called amyloids. Recent evidence suggests that these structures include both large, insoluble fibrils and smaller, prefibrillar structures, such as dimers, oligomers, and protofibrils. Recently, focus has shifted
E L Paley et al.
Experimental cell research, 195(1), 66-78 (1991-07-01)
Bovine kidney cell lines resistant to tryptamine and tryptophanol (tryptophan analogs) were selected. The content of tryptophanyl-tRNA synthetase (WRS, EC 6.1.1.2) was assayed by measuring the binding of monospecific polyclonal antibodies to the 35S-labeled enzyme in detergent-soluble and -insoluble forms
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique