412546
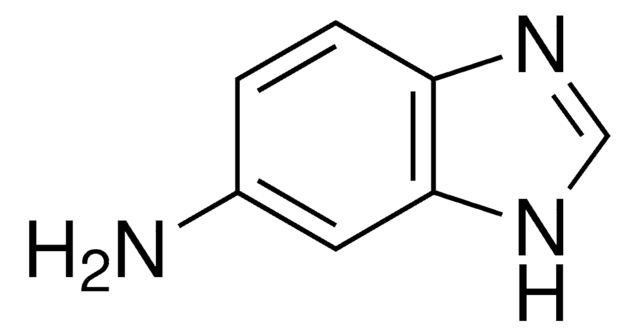
2-Amino-1-methylbenzimidazole
95%
Se connecterpour consulter vos tarifs contractuels et ceux de votre entreprise/organisme
About This Item
Formule empirique (notation de Hill) :
C8H9N3
Numéro CAS:
Poids moléculaire :
147.18
Numéro MDL:
Code UNSPSC :
12352100
eCl@ss :
32151902
ID de substance PubChem :
Nomenclature NACRES :
NA.22
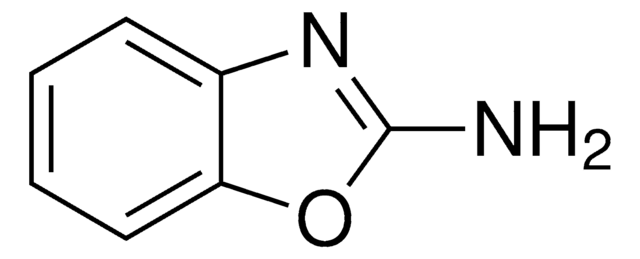
Produits recommandés
Essai
95%
Chaîne SMILES
Cn1c(N)nc2ccccc12
InChI
1S/C8H9N3/c1-11-7-5-3-2-4-6(7)10-8(11)9/h2-5H,1H3,(H2,9,10)
Clé InChI
XDFZKQJLNGNJAN-UHFFFAOYSA-N
Catégories apparentées
Description générale
The carbonyl-scavenging ability of 2-amino-1-methylbenzimidazole has been investigated. Mechanism of Menshutkin reaction between 2-amino-1-methylbenzimidazole and iodomethane has been studied in gas phase and in liquid acetonitrile. It is reported to form adducts with natural allyl, phenethyl, and benzyl isothiocyanates.
Application
2-Amino-1-methylbenzimidazole may be used for the preparation of 2- or 3-carboxy-4H-pyrimido[2,1-b]-benzazol-4-ones and novel functionalized spiropyran′s derivatives of 2H-1,3-benzoxazinone series.
Mention d'avertissement
Warning
Mentions de danger
Conseils de prudence
Classification des risques
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Organes cibles
Respiratory system
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Équipement de protection individuelle
dust mask type N95 (US), Eyeshields, Gloves
Faites votre choix parmi les versions les plus récentes :
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
André Melo et al.
The journal of physical chemistry. B, 110(4), 1877-1888 (2006-02-14)
The quaternization reaction between 2-amino-1-methylbenzimidazole and iodomethane was investigated in the gas phase and in liquid acetonitrile. Both experimental and theoretical techniques were used in this study. In the experimental part of this work, accurate second-order rate constants were obtained
Synthesis and antiallergic activity of some acidic derivatives of 4H-pyrimido[2,1-b]benzazol-4-ones.
J J Wade et al.
Journal of medicinal chemistry, 26(4), 608-611 (1983-04-01)
Reactions of 2-aminobenzothiazole, 2-aminobenzoxazole, and 2-amino-1-methylbenzimidazole with dimethyl aminofumarate (DMAF) or diethyl ethoxymethylenemalonate (DEEM) led to 2- or 3-carboxy-4H-pyrimido[2,1-b]-benzazol-4-ones, respectively. Subsequent derivatization of these carboxylic acids gave the corresponding tetrazolylcarboxamides and tetrazoles. These acidic compounds were tested in the rat
Synthesis and structural characterization of novel 2-benzimidazolylthioureas: adducts of natural isothiocyanates and 2-amino-1-methylbenzimidazole.
Smiechowska A, et al.
Structural Chemistry, 21(5), 955-964 (2010)
Antony O Bulanov et al.
Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy, 71(3), 1146-1152 (2008-06-14)
Six novel functionalized spiropyran's derivatives of 2H-1,3-benzoxazinone series were synthesized by introducing the substituents with chelating ability into 2H-chromene part of the 8'-formyl-7'-hydroxy-3-methyl-4-oxo-3,4-dihydro-2H-1,3-benzoxazine-2-spiro-2'-[2H]-chromene (I) by condensation with 2-aminophenol, 2-amino-4-methylphenol, 2-amino-4-nitrophenol, 2-amino-1-methylbenzimidazole, 4-amino-4H-1,2,4-triazole, N-(4-aminophenyl)acetamide. (1)H NMR, UV/vis, IR spectroscopy combined with
Francisco J Hidalgo et al.
Journal of agricultural and food chemistry, 62(49), 12045-12051 (2014-11-25)
The carbonyl-scavenging ability of 2-amino-1-methylbenzimidazole (AMBI) and the heterocyclic aromatic amine 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) was investigated in an attempt to identify new routes that can modify the carbonyl content of foods. The reaction of both AMBI and PhIP with 2-alkenals, 2,4-alkadienals
Global Trade Item Number
| Référence | GTIN |
|---|---|
| 412546-1G | 4061826663202 |
| 412546-5G | 4061832075952 |
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique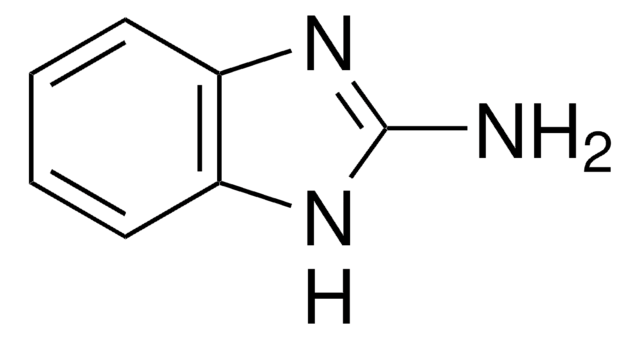



![1′,3′-Dihydro-1′,3′,3′-trimethyl-6-nitrospiro[2H-1-benzopyran-2,2′-(2H)-indole] 98%](/deepweb/assets/sigmaaldrich/product/structures/503/745/147ecd2c-44b9-46e9-a8c9-bff9a2577218/640/147ecd2c-44b9-46e9-a8c9-bff9a2577218.png)