378674
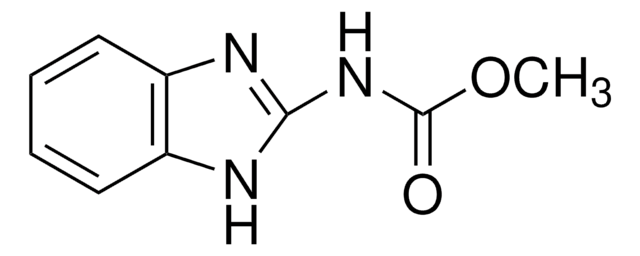
Carbendazim
97%
Synonyme(s) :
2-benzimidazolecarbamate de méthyle
About This Item
Produits recommandés
Niveau de qualité
Pureté
97%
Forme
powder
Pf
>300 °C (lit.)
Solubilité
pyridine: soluble 1%, clear, very faintly brownish-yellow
Groupe fonctionnel
amine
Chaîne SMILES
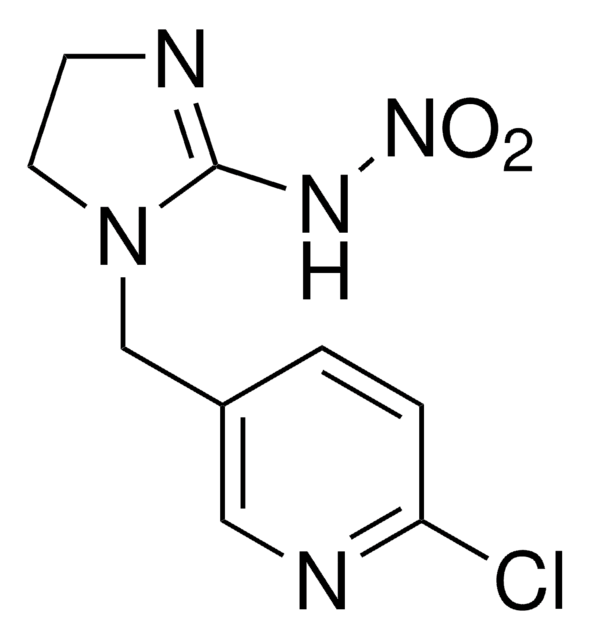
COC(=O)Nc1nc2ccccc2[nH]1
InChI
1S/C9H9N3O2/c1-14-9(13)12-8-10-6-4-2-3-5-7(6)11-8/h2-5H,1H3,(H2,10,11,12,13)
Clé InChI
TWFZGCMQGLPBSX-UHFFFAOYSA-N
Vous recherchez des produits similaires ? Visite Guide de comparaison des produits
Description générale
Application
- As positive control in direct contact assay (mycelial radial growth inhibition assay).
- As pesticide and its effect on the transcription responses of Enchytraeus albidus have been studied.
- As broad-spectrum antifungal compound, to study the antibacterial action of anti-tuberculosis drugs against Mycobacterium tuberculosis in the Dubos broth culture medium.
Mention d'avertissement
Danger
Mentions de danger
Conseils de prudence
Classification des risques
Aquatic Acute 1 - Aquatic Chronic 1 - Muta. 1B - Repr. 1B - Skin Sens. 1
Code de la classe de stockage
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Équipement de protection individuelle
Eyeshields, Gloves, type P3 (EN 143) respirator cartridges
Faites votre choix parmi les versions les plus récentes :
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique