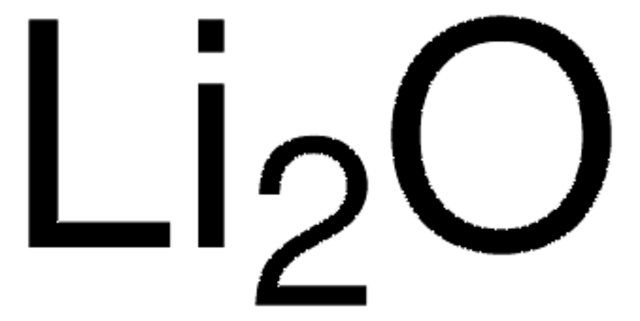237965
Lithium fluoride
powder, -300 mesh
Synonyme(s) :
Fluorolithium
About This Item
Produits recommandés
Qualité
for analytical purposes
Forme
powder
Caractéristiques du produit alternatif plus écologique
Design for Energy Efficiency
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
Taille des particules
-300 mesh
Pf
845 °C (lit.)
Densité
2.64 g/mL at 25 °C (lit.)
Autre catégorie plus écologique
Chaîne SMILES
[Li+].[F-]
InChI
1S/FH.Li/h1H;/q;+1/p-1
Clé InChI
PQXKHYXIUOZZFA-UHFFFAOYSA-M
Vous recherchez des produits similaires ? Visite Guide de comparaison des produits
Description générale
We are committed to bringing you Greener Alternative Products, which adhere to one or more of The 12 Principles of Greener Chemistry. This product has been enhanced for energy efficiency. Click here for more information.
Application
Lithium fluoride (LiF) can be used as a sintering aid that facilitates the etching of spinel particles for the degradation of magnesium aluminum spinel. It can be used in the preparation of carbon-iron LiF nanocomposite based electrode materials for lithium-ion batteries. It can also form thin interlayers on the counter electrode to enhance the fill factor and stabilize the high open-circuit voltages for polymer solar cells.
Mention d'avertissement
Warning
Mentions de danger
Conseils de prudence
Classification des risques
Acute Tox. 4 Oral - Eye Irrit. 2
Risques supp
Code de la classe de stockage
6.1D - Non-combustible acute toxic Cat.3 / toxic hazardous materials or hazardous materials causing chronic effects
Classe de danger pour l'eau (WGK)
WGK 2
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Équipement de protection individuelle
Eyeshields, Faceshields, Gloves, type P2 (EN 143) respirator cartridges
Faites votre choix parmi les versions les plus récentes :
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Articles
Professor Gogotsi and Dr. Shuck introduce MXenes: a promising family of two-dimensional materials with a unique combination of high conductivity, hydrophilicity, and extensive tunability.
Research and development of solid-state lithium fast-ion conductors is crucial because they can be potentially used as solid electrolytes in all-solid-state batteries, which may solve the safety and energy-density related issues of conventional lithium-ion batteries that use liquid (farmable organic) electrolytes.
Lithium-Ion Battery Performance: Dependence on Material Synthesis and Post‑Treatment Methods
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique