PHL89157
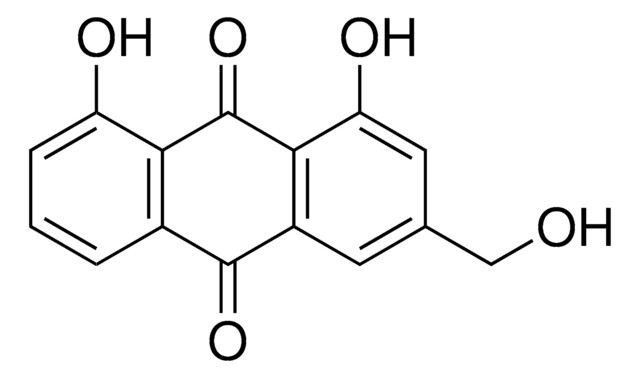
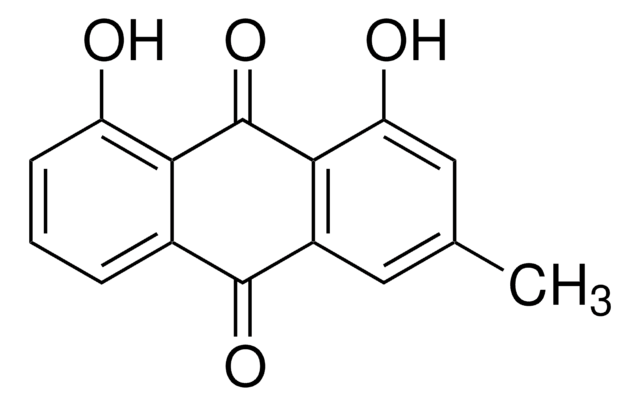
Aloe-emodin
phyproof® Reference Substance
Synonym(s):
1,8-Dihydroxy 3-hydroxymethylanthraquinone, 1,8-Dihydroxy-3-(hydroxymethyl)anthraquinone, 3-Hydroxymethylchrysazine, Rhabarberone
About This Item
Recommended Products
grade
primary reference standard
product line
phyproof® Reference Substance
Assay
≥95.0% (HPLC)
form
solid
manufacturer/tradename
PhytoLab
technique(s)
gas chromatography (GC): suitable
liquid chromatography (LC): suitable
application(s)
cannabis testing
format
neat
SMILES string
OCc1cc(O)c2C(=O)c3c(O)cccc3C(=O)c2c1
InChI
1S/C15H10O5/c16-6-7-4-9-13(11(18)5-7)15(20)12-8(14(9)19)2-1-3-10(12)17/h1-5,16-18H,6H2
InChI key
YDQWDHRMZQUTBA-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Application
Biochem/physiol Actions
Other Notes
Legal Information
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Global Trade Item Number
| SKU | GTIN |
|---|---|
| PHL89157-20MG |
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service