PHL80431
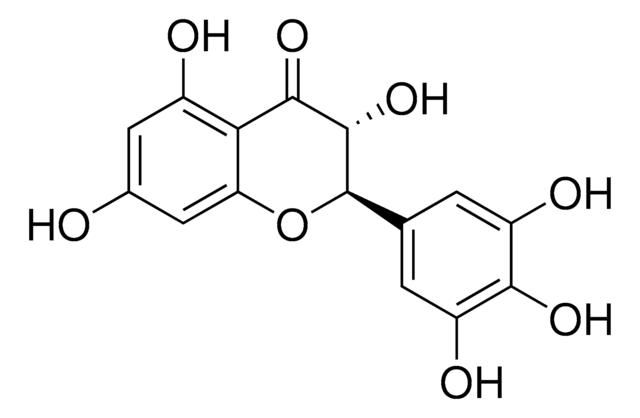
Dihydromyricetin
phyproof® Reference Substance
Synonym(s):
(2R,3R)-3,5,7-Trihydroxy-2-(3,4,5-trihydroxyphenyl)-2,3-dihydrochromen-4-one, 3,3′,4′,5,5′,7-Hexahydroxyflavanone, Ampelopsin, Ampeloptin, DHM
About This Item
Recommended Products
grade
primary reference standard
product line
phyproof® Reference Substance
Assay
≥95.0% (HPLC)
form
solid
manufacturer/tradename
PhytoLab
SMILES string
O=C1C2=C(O)C=C(O)C=C2O[C@H](C3=CC(O)=C(C(O)=C3)O)[C@H]1O
InChI
1S/C15H12O8/c16-6-3-7(17)11-10(4-6)23-15(14(22)13(11)21)5-1-8(18)12(20)9(19)2-5/h1-4,14-20,22H/t14-,15+/m0/s1
InChI key
KJXSIXMJHKAJOD-LSDHHAIUSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Application
- Hovenia dulcis: a Chinese medicine that plays an essential role in alcohol-associated liver disease. This review discusses the role of Hovenia dulcis, from which dihydromyricetin is derived, in treating alcohol-associated liver conditions, highlighting its mechanisms and therapeutic potentials (He YX, Liu MN, Wang YY, et al. 2024).
- Dihydromyricetin ameliorates hepatic steatosis and insulin resistance via AMPK/PGC-1α and PPARα-mediated autophagy pathway. This study explores how dihydromyricetin influences liver health, particularly in hepatic steatosis and insulin resistance, offering insights into its mechanisms through autophagy pathways (Yang Y, Qiu W, Xiao J, et al. 2024).
- Identification of dihydromyricetin as a natural DNA methylation inhibitor with rejuvenating activity in human skin. Research identifies dihydromyricetin′s potential anti-aging effects on human skin by modulating DNA methylation, which could contribute to its broader use in dermatological products (Falckenhayn C, Bienkowska A, Söhle J, et al. 2023).
- Dihydromyricetin reverses capecitabine-induced peripheral myelin dysfunction through modulation of oxidative stress. This article provides evidence of dihydromyricetin′s protective effects against peripheral myelin damage due to oxidative stress, relevant in the treatment of certain neuropathies (Fang J, Lou S, Zhou X, et al. 2024).
- The Molecular Mechanism Underlying the Therapeutic Effect of Dihydromyricetin on Type 2 Diabetes Mellitus Based on Network Pharmacology, Molecular Docking, and Transcriptomics. This comprehensive study details the molecular interactions and pathways through which dihydromyricetin could affect type 2 diabetes, providing a foundation for its application in metabolic disorder treatments (Wen X, Lv C, Zhou R, et al. 2024).
Biochem/physiol Actions
Legal Information
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service