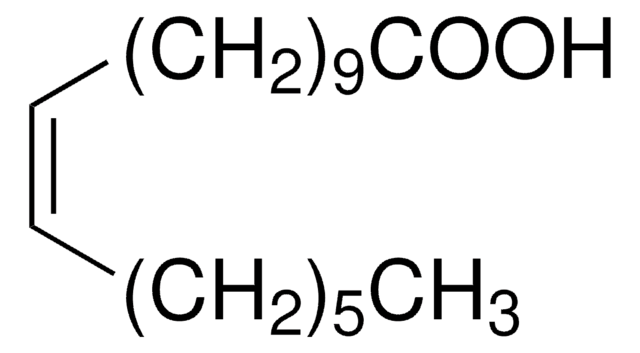69301
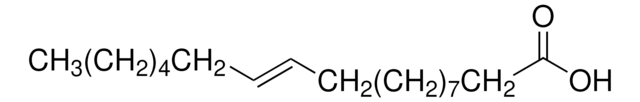
cis-Vaccenic acid
analytical standard
Synonym(s):
octadec-11(cis)-enoic acid, cis-11-Octadecenoic acid
About This Item
Recommended Products
grade
analytical standard
Assay
≥97.0% (HPLC)
form
liquid
shelf life
limited shelf life, expiry date on the label
impurities
≤0.5% trans-isomer
refractive index
n20/D 1.459 (lit.)
bp
150 °C/0.03 mmHg (lit.)
mp
14-15 °C (lit.)
density
0.887 g/mL at 25 °C (lit.)
application(s)
clinical testing
storage temp.
−20°C
SMILES string
CCCCCC\C=C/CCCCCCCCCC(O)=O
InChI
1S/C18H34O2/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-18(19)20/h7-8H,2-6,9-17H2,1H3,(H,19,20)/b8-7-
InChI key
UWHZIFQPPBDJPM-FPLPWBNLSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Application
- Quantification of the analyte and its photodegradation products in marine samples using gas chromatography coupled to electron impact mass spectrometry (GC-EIMS). The photo-oxidation of the lipid components of the two strains of aerobic photoheterotrophic bacteria, Erythrobacter sp. strain NAP1 and Roseobacter-related isolate COL2P is studied and the results obtained after irradiation of axenic and nonaxenic cultures of the diatom, Skeletonema costatum is compared.
- Quantification of the analyte in the methanolic extract of the leaves of Lepidium sativum using gas chromatography coupled to mass spectrometry (GC-MS).
- Quantification of the analyte in methanolic extract of Rosmarinus oficinalis leaves using gas chromatography coupled to mass spectrometry and Fourier transform infrared spectroscopy.
Recommended products
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
446.0 °F - closed cup
Flash Point(C)
230 °C - closed cup
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service