General description
As determined by ELISA and dot blot, the antibody reacts specifically with phosphorylated threonine, both as free amino acid or conjugated to carriers such as BSA or KLH. No cross-reactivity is observed with non-phosphorylated threonine, phosphoserine, phosphotyrosine, AMP or ATP. This antibody has been used in immunoblotting for the localization of some phosphothreonine-containing proteins. Certain proteins known to contain phosphorylated threonine may not be recognized by this antibody due to steric hindrance of the recognition site.
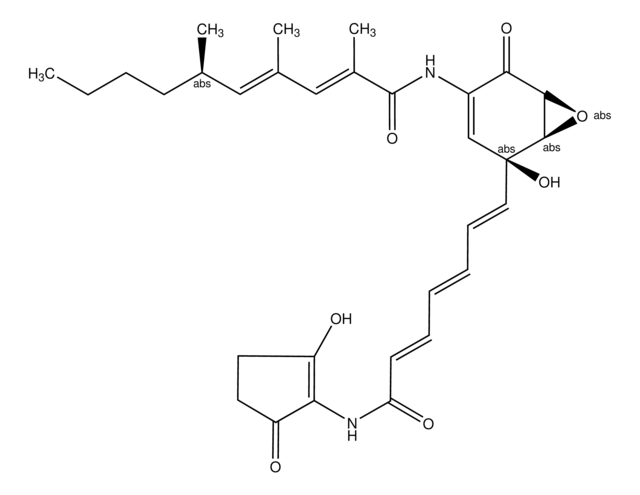
Monoclonal Anti-Phosphothreonine (mouse IgG2b isotype) is derived from the hybridoma produced by the fusion of mouse myeloma cells (NS1) and splenocytes from BALB/c mice immunized with phosphothreonine conjugated to keyhole limpet hemocyanin (KLH).
Mouse monoclonal clone PTR-8 anti-Phosphothreonine antibody reacts with phosphorylated threonine both as a free amino acid or when conjugated to carriers such as BSA or KLH, using ELISA and dot blot. It does not react with nonphosphorylated threonine, phosphorylated tyrosine or serine, AMP or ATP.
Specificity
Monoclonal Anti-Phosphothreonine reacts with phosphorylated threonine both as a free amino acid or when conjugated to carriers such as BSA or KLH, using ELISA and dot blot. It does not react with nonphosphorylated threonine, phosphorylated tyrosine or serine, AMP or ATP.
Immunogen
phosphothreonine conjugated to keyhole limpet hemocyanin (KLH).
Application
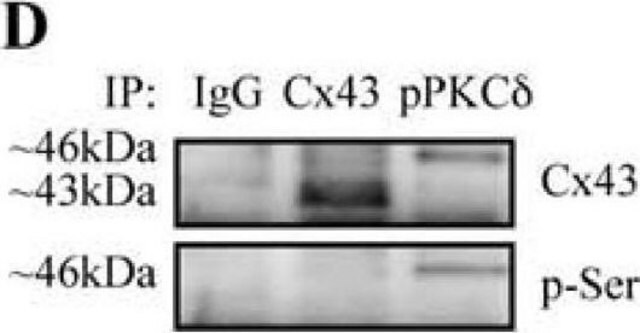
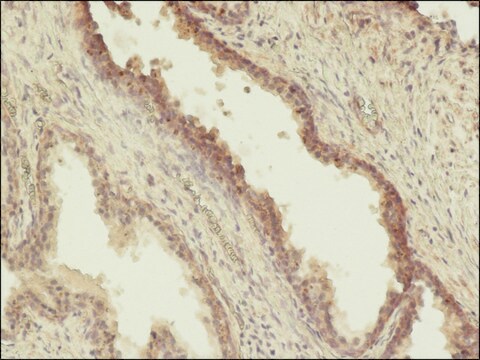
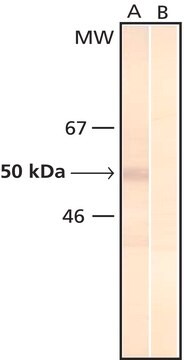
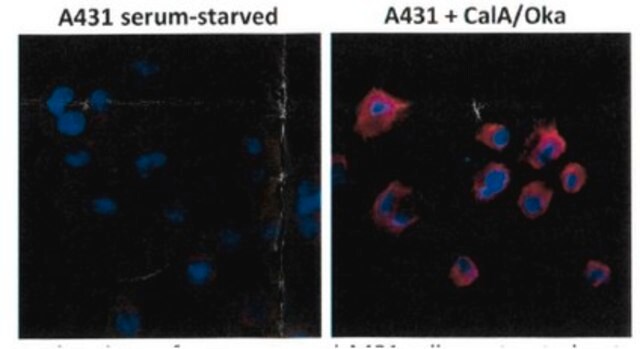
Monoclonal Anti-Phosphothreonine antibody produced in mouse has been used in enzyme-linked immunosorbent assay (ELISA), dot blot, immunoprecipitation, immunocytochemistry and immunoblotting. It has also been used in Akt kinase assay to detect phosphorylation status on serine and threonine residue in the wild type or mutant type δ-catenin.
Biochem/physiol Actions
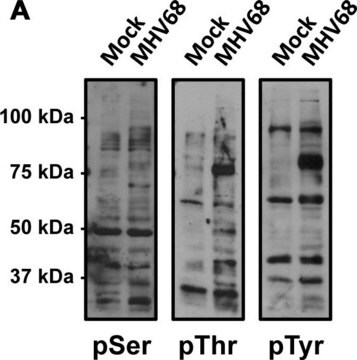
Protein phosphorylation and dephosphorylation are basic mechanisms for the modification of protein function in eukaryotic cells. Phosphorylation is a rare post-translational event in normal tissue. Various activation processes are mediated through phosphotyrosine, phosphoserine or phosphothreonine (p-Tyr/p-Ser/p-Thr). Many different mitogenic systems, such as the epidermal growth factor (EGF), platelet derived growth factor (PDGF) and insulin receptor systems contain Tyr/Ser/Thr kinase domains that autophosphorylate specific Tyr/Ser/Thr residues upon binding of their ligands. T cell antigen receptor complex or receptors for some hemopoietic growth factors may stimulate associated kinases, and cells transformed by viral oncogenes contain elevated levels of phosphorylated Tyr/Ser/Thr.
Physical form
Solution in 0.01 M phosphate buffered saline, pH 7.4, containing 15 mM sodium azide.
Storage and Stability
For continuous use, store at 2-8 °C for up to one month. For extended storage, freeze at -20 °C in working aliquots. Repeated freezing and thawing, or storage in "frost-free" freezers, is not recommended. If slight turbidity occurs upon prolonged storage, clarify the solution by centrifugation before use. Working dilution samples should be discarded if not used within 12 hours.
Disclaimer
Unless otherwise stated in our catalog or other company documentation accompanying the product(s), our products are intended for research use only and are not to be used for any other purpose, which includes but is not limited to, unauthorized commercial uses, in vitro diagnostic uses, ex vivo or in vivo therapeutic uses or any type of consumption or application to humans or animals.