P3818
Protein Disulfide Isomerase from bovine liver
≥100 units/mg protein, lyophilized powder
Synonym(s):
PDI
About This Item
Recommended Products
form
lyophilized powder
Quality Level
specific activity
≥100 units/mg protein
mol wt
107 kDa
composition
Protein, ~10% Lowry
storage temp.
−20°C
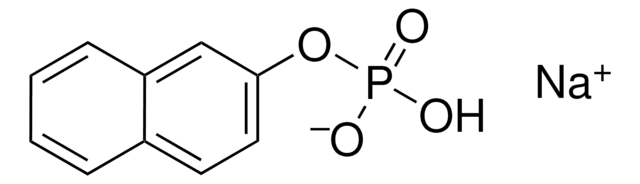
SMILES string
[S](=O)(=O)(ON)c1c(cc(c(c1)C(=O)O)Cl)Cl
InChI
1S/C7H5Cl2NO5S/c8-4-2-5(9)6(16(13,14)15-10)1-3(4)7(11)12/h1-2H,10H2,(H,11,12)
InChI key
DHUYKLYJBKXDBM-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
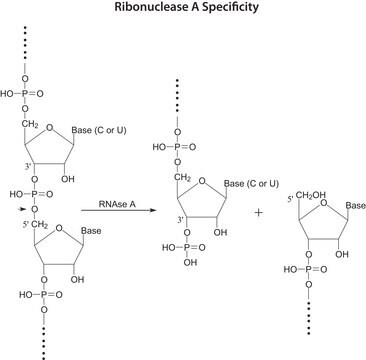
General description
Application
- to study the functional role of PDI in parasite infection and the interaction between macrophage PDI and L. chagasi
- in the in vitro translation reaction for the generation of disulfide bonds
- in insulin-disulfide reduction assay and peptide binding assay
- as a positive control in thiol-disulfide oxidoreductase activity assay
Biochem/physiol Actions
Packaging
Physical properties
Unit Definition
Physical form
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Articles
Oxidative stress is mediated, in part, by reactive oxygen species produced by multiple cellular processes and controlled by cellular antioxidant mechanisms such as enzymatic scavengers or antioxidant modulators. Free radicals, such as reactive oxygen species, cause cellular damage via cellular.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service