M7773
Monoclonal Anti-Myoglobin antibody produced in mouse
clone MG-1, ascites fluid
Synonym(s):
Anti-PVALB
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Recommended Products
biological source
mouse
Quality Level
conjugate
unconjugated
antibody form
ascites fluid
antibody product type
primary antibodies
clone
MG-1, monoclonal
contains
15 mM sodium azide
species reactivity
human
technique(s)
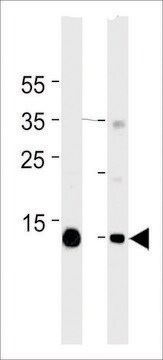
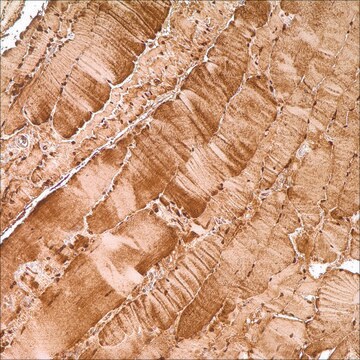
immunohistochemistry (formalin-fixed, paraffin-embedded sections): 1:400 using human skeletal muscle tissue
indirect ELISA: 1:10,000
isotype
IgG1
UniProt accession no.
shipped in
dry ice
storage temp.
−20°C
target post-translational modification
unmodified
Gene Information
human ... MB(4151)
Related Categories
General description
Myoglobin is a hemoprotein that regulates the storage and diffusion of oxygen in heart and skeletal muscles. Additionally, this protein also protects the tissues from oxidative damage by controlling the levels of reactive oxygen species and nitric oxide. Thus, myoglobin has been implicated in regulating nitric oxide and oxygen levels in the mitochondrial compartments of skeletal muscle and cardiac cells. Monoclonal Anti-Myoglobin antibody is specific for myoglobin and stains human skeletal muscles. The product does not cross-react with hemoglobin.
Myoglobin is composed of a 153 amino acid long polypeptide and heme group. This protein is encoded by the gene MB mapped to human chromosome 22q12.3. It is a unit of 20S core proteasome complex. Myoglobin is localized to the skeletal and cardiac muscle.
Immunogen
Purified human skeletal muscle myoglobin.
Application
Monoclonal Anti-Myoglobin antibody is suitable for use in western blot and protein arrays.
Biochem/physiol Actions
Myoglobin participates in proteases mediated degradation of intracellular proteins Upon damage to the muscle cell due to infarction of a coronary artery, neurological trauma, infection or tumor processes, myoglobin escapes to the environment and can be found in plasma using sensitive assays.
Disclaimer
Unless otherwise stated in our catalog or other company documentation accompanying the product(s), our products are intended for research use only and are not to be used for any other purpose, which includes but is not limited to, unauthorized commercial uses, in vitro diagnostic uses, ex vivo or in vivo therapeutic uses or any type of consumption or application to humans or animals.
Not finding the right product?
Try our Product Selector Tool.
Storage Class Code
10 - Combustible liquids
WGK
nwg
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Myoglobin
Redei GP
Encyclopedia of Genetics, Genomics, Proteomics, and Informatics, 1314-1314 (2008)
U B Hendgen-Cotta et al.
The Journal of experimental biology, 213(Pt 16), 2734-2740 (2010-08-03)
For more than 100 years, myoglobin has been among the most extensively studied proteins. Since the first comprehensive review on myoglobin function as a dioxygen store by Millikan in 1939 and the discovery of its structure 50 years ago, multiple
D J Marcinek et al.
American journal of physiology. Regulatory, integrative and comparative physiology, 280(4), R1123-R1133 (2001-03-15)
Myoglobin (Mb) buffers intracellular O2 and facilitates diffusion of O2 through the cell. These functions of Mb will be most effective when intracellular PO2 is near the partial pressure of oxygen at which Mb is half saturated (P50) of the
Joy G Ghosh et al.
Protein science : a publication of the Protein Society, 14(3), 684-695 (2005-02-22)
Protein pin array technology was used to identify subunit-subunit interaction sites in the small heat shock protein (sHSP) alphaB crystallin. Subunit-subunit interaction sites were defined as consensus sequences that interacted with both human alphaA crystallin and alphaB crystallin. The human
George A Ordway et al.
The Journal of experimental biology, 207(Pt 20), 3441-3446 (2004-09-02)
Myoglobin is a cytoplasmic hemoprotein, expressed solely in cardiac myocytes and oxidative skeletal muscle fibers, that reversibly binds O2 by its heme residue, a porphyrin ring:iron ion complex. Since the initial discovery of its structure over 40 years ago, wide-ranging
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service