All Photos(3)
About This Item
Empirical Formula (Hill Notation):
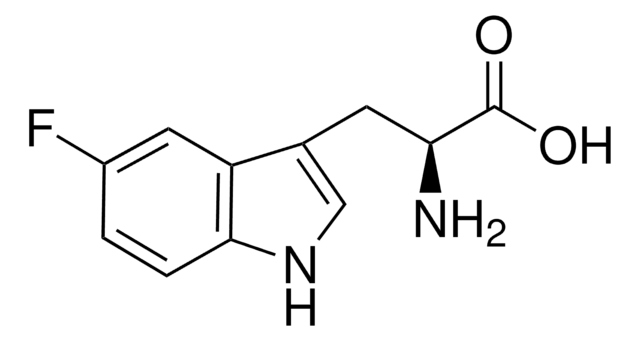
C12H14N2O3
CAS Number:
Molecular Weight:
234.25
Beilstein:
26781
EC Number:
MDL number:
UNSPSC Code:
12352209
PubChem Substance ID:
NACRES:
NA.26
Recommended Products
Product Name
5-Methoxy-DL-tryptophan,
Assay
≥98% (TLC)
Quality Level
form
crystalline
color
white to light beige
mp
258-261 °C (dec.) (lit.)
application(s)
peptide synthesis
storage temp.
−20°C
SMILES string
COc1ccc2[nH]cc(CC(N)C(O)=O)c2c1
InChI
1S/C12H14N2O3/c1-17-8-2-3-11-9(5-8)7(6-14-11)4-10(13)12(15)16/h2-3,5-6,10,14H,4,13H2,1H3,(H,15,16)
InChI key
KVNPSKDDJARYKK-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Biochem/physiol Actions
5-Methoxy-DL-tryptophan is an amino acid derivative.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Anika Kremer et al.
Applied microbiology and biotechnology, 79(6), 951-961 (2008-05-16)
Recently, a gene for a 7-dimethylallyltryptophan synthase (7-DMATS) was identified in Aspergillus fumigatus and its enzymatic function was proven biochemically. In this study, the behaviour of 7-DMATS towards aromatic substrates was investigated and compared with that of the 4-dimethylallyltryptophan synthase
J van Benthem et al.
Journal of neural transmission, 67(1-2), 147-162 (1986-01-01)
Testes weight, plasma FSH and LH concentration and pineal methylating capacity were compared in hamsters housed under either long (LD14:10) or short (LD8:16) photoperiods. Hamsters housed for 14 weeks under short photoperiod showed gonadal atrophy, which was complete after 6
D J Morton
Journal of pineal research, 4(1), 7-11 (1987-01-01)
Pineal glands were incubated in the presence of [3H] methoxytryptophan with and without methoxamine, epinephrine, and norepinephrine. The beta-adrenoceptor-stimulated pineal glands were capable of converting methoxytryptophan to methoxytryptamine, melatonin, methoxyindole acetic acid, and methoxytryptophol, albeit in small quantities. Only methoxyindole
J van Benthem et al.
Journal of neural transmission, 61(3-4), 219-237 (1985-01-01)
Until now the day/night and seasonal rhythmicity in the synthesis of 5-methoxyindoles (MI) is thought to be regulated by environmental factors, especially photoperiod and temperature. Endogenous factors are also implicated in the generation of N-acetyltransferase and hydroxyindole-O-methyltransferase activity rhythms. In
G L Brammer
Life sciences, 55(10), 775-787 (1994-01-01)
Markedly increased melatonin levels in plasma have been observed in response to tryptophan administration. This post-tryptophan melatonin increase has been attributed to the duodenum. Because extra-pineal sources of melatonin may be important in interpreting the meaning of altered melatonin production
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service