H7890
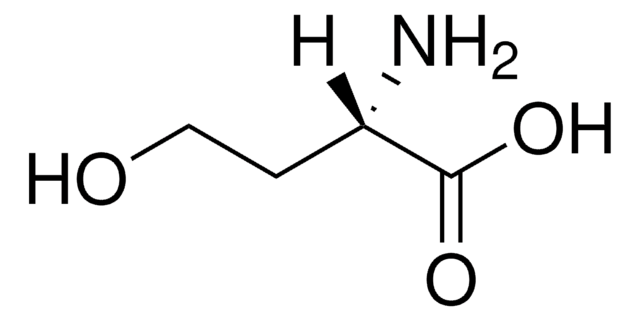
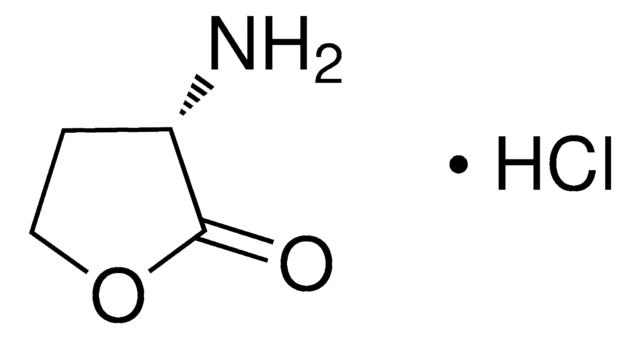
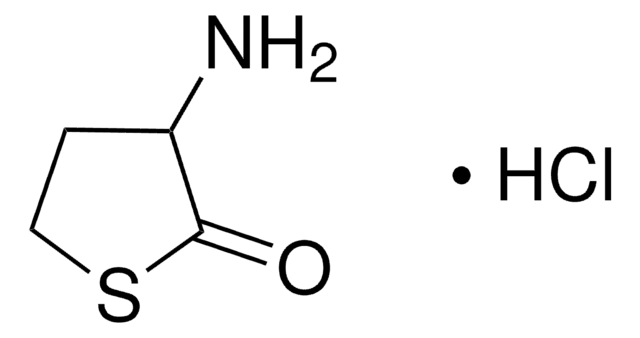
L-Homoserine lactone hydrochloride
suitable for ligand binding assays
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Empirical Formula (Hill Notation):
C4H7NO2 · HCl
CAS Number:
Molecular Weight:
137.56
MDL number:
UNSPSC Code:
12352209
PubChem Substance ID:
NACRES:
NA.26
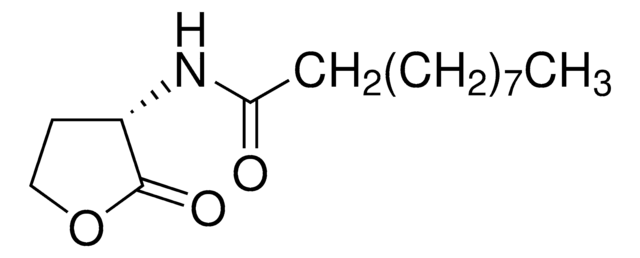
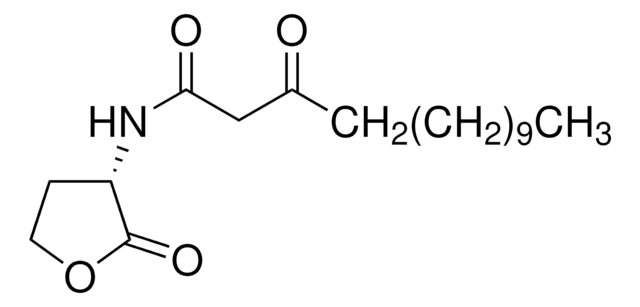
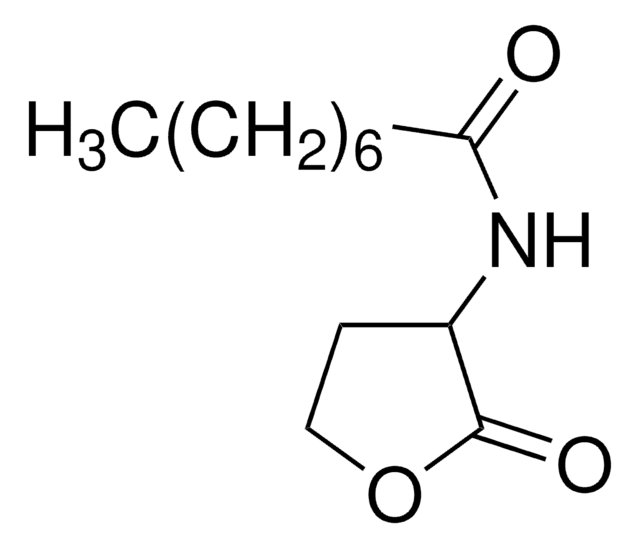
Recommended Products
Product Name
L-Homoserine lactone hydrochloride,
form
powder
Quality Level
technique(s)
ligand binding assay: suitable
color
white
storage temp.
−20°C
SMILES string
Cl.N[C@H]1CCOC1=O
InChI
1S/C4H7NO2.ClH/c5-3-1-2-7-4(3)6;/h3H,1-2,5H2;1H/t3-;/m0./s1
InChI key
XBKCXPRYTLOQKS-DFWYDOINSA-N
General description
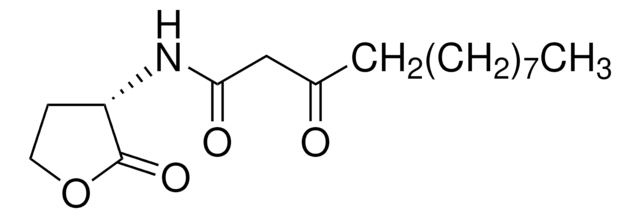
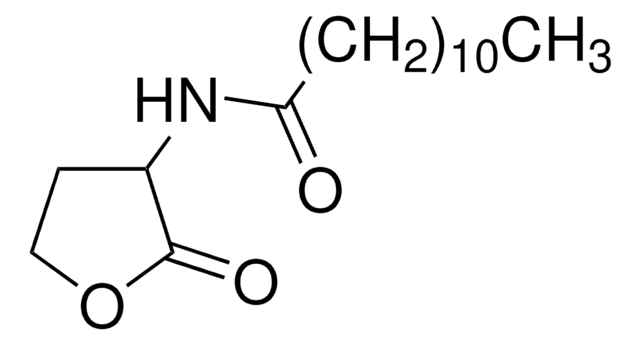
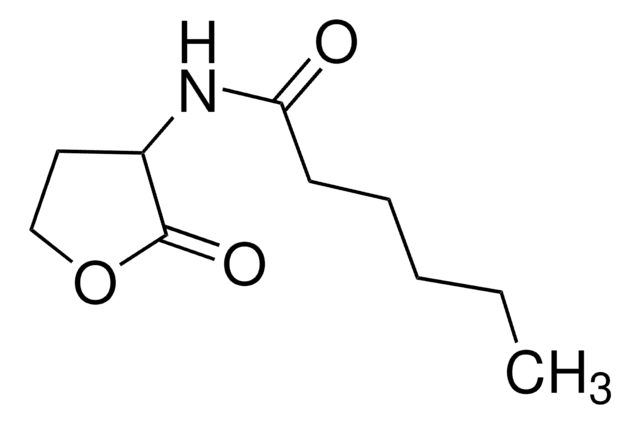
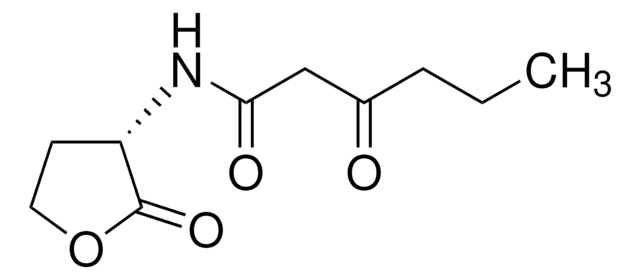
L-Homoserine lactone (HSL), also called acyl-HSL, comprises a homoserine lactone ring and a fatty acyl side. The levels of acyl-HSL in bacteria is dictated by the availability of the substrates and acyl-homoserine lactones synthase.
Application
L-Homoserine lactone hydrochloride has been used as an inhibitor for arterial smooth muscle contraction.
Biochem/physiol Actions
L-Homoserine lactone inhibition reduces virulence factors and halts inflammation and tissue damage. In Pseudomonas aeruginosa it controls virulence factor production and biofilm formation. In Agrobacterium tumefaciens, it is essential for conjugal transfer.
Acylated L-Homoserine lactone(s) are used to study bacterial quorum-sensing signaling mechanisms.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Defining the structure and function of acyl-homoserine lactone autoinducers
Churchill MEA, et al.
Methods in Molecular Biology, 692(23), 159-171 (2011)
Long-chain acyl-homoserine lactone quorum-sensing regulation of Rhodobacter capsulatus gene transfer agent production
Schaefer AL, et al.
Journal of Bacteriology, 184(23), 6515-6521 (2002)
The Pseudomonas aeruginosa quorum-sensing signal molecule, N-(3-oxododecanoyl)-l-homoserine lactone, inhibits porcine arterial smooth muscle contraction
Lawrence RN, et al.
British Journal of Pharmacology, 128(4), 845-848 (1999)
Adam Schikora et al.
Plant physiology, 157(3), 1407-1418 (2011-09-24)
Pathogenic and symbiotic bacteria rely on quorum sensing to coordinate the collective behavior during the interactions with their eukaryotic hosts. Many Gram-negative bacteria use N-acyl-homoserine lactones (AHLs) as signals in such communication. Here we show that plants have evolved means
Inhibiting N-acyl-homoserine lactone synthesis and quenching Pseudomonas quinolone quorum sensing to attenuate virulence
Chan KG, et al.
Frontiers in Microbiology, 6, 1173-1173 (2015)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service