D9779
DL-Dithiothreitol
for molecular biology, ≥98% (HPLC), ≥99% (titration)
Synonym(s):
(±)-Dithiothreitol, rac-Dithiothreitol, Dithiothreitol, threo-1,4-Dimercapto-2,3-butanediol, Cleland’s reagent, DTT
About This Item
Recommended Products
grade
for molecular biology
Quality Level
Assay
≥98% (HPLC)
≥99% (titration)
form
powder
reaction suitability
reagent type: reductant
mp
41-44 °C (lit.)
solubility
H2O: 50 mg/mL
cation traces
heavy metals (as Pb): ≤5 ppm
suitability
suitable for molecular biology
foreign activity
DNase, RNase, protease, none detected
storage temp.
2-8°C
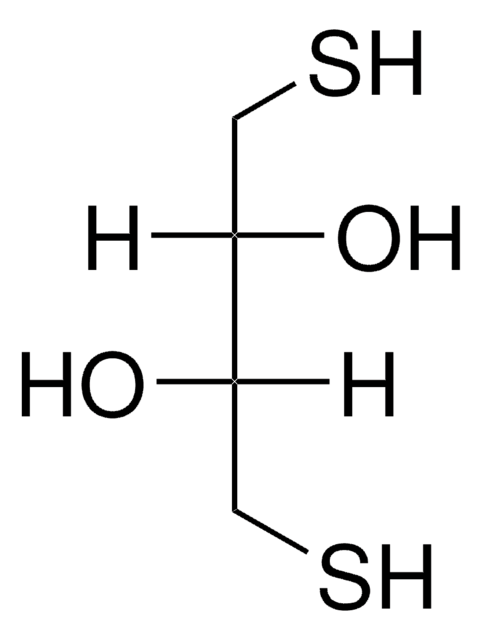
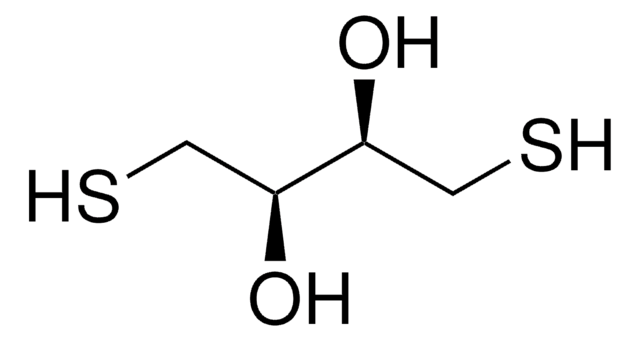
SMILES string
O[C@H](CS)[C@H](O)CS
InChI
1S/C4H10O2S2/c5-3(1-7)4(6)2-8/h3-8H,1-2H2/t3-,4-/m1/s1
InChI key
VHJLVAABSRFDPM-QWWZWVQMSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Application
It has been used:
- as a component for protein extraction in western blot analysis
- to prepare sample lysis buffer for quantitative mass spectroscopy
- as a kinase buffer component for enzyme-linked immunosorbent assay (ELISA)
Biochem/physiol Actions
Features and Benefits
- Suitable for molecular biology
- RNase, DNase, Exonuclease, and Protease-free
- High purity (HPLC ≥98%)
- No heavy metal ≤5ppm
Other Notes
comparable product
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Dam. 1 - Skin Irrit. 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Protocols
Follow this DDT reduction protocol to reduce disulfide bonds in thiol-modified oligonucleotides, thereby avoiding this source of oligo dimer formation.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service