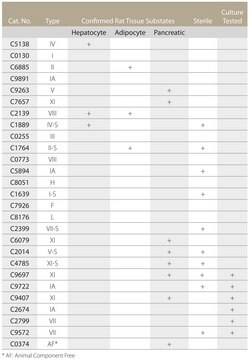
C9722
Collagenase from Clostridium histolyticum
lyophilized powder (from 0.2μm filtered solution), 0.5-5.0 FALGPA units/mg solid, suitable for cell culture
Synonym(s):
Clostridiopeptidase A
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Recommended Products
biological source
Clostridium histolyticum
Quality Level
sterility
0.2 μm filtered
form
lyophilized powder (from 0.2μm filtered solution)
specific activity
0.5-5.0 FALGPA units/mg solid
concentration
1 mg/mL
technique(s)
cell culture | mammalian: suitable
single cell analysis: suitable
pH
7.4
shipped in
ambient
storage temp.
−20°C
Looking for similar products? Visit Product Comparison Guide
Application
This product is suitable for the disaggregation of human tumor, mouse kidney, human adult and fetal brain, lung and many other epithelia tissues. It has also been shown to be effective in liver and kidney perfusion studies, digestion of pancreas, isolation of nonparenchymal rat liver cells and hepatocyte preparation. Collagenase has also been used in the preparation of arterial tissue for the study of Advanced Glycosylation End Products. This enzyme has been tested for the release of heptatocytes at a concentration of approximately 1 mg/mL. Concentrations for digestion range from 0.1 to 5 mg/mL.
Suitable for use in preparation of single cell suspension for sequencing.
Suitable for use in preparation of single cell suspension for sequencing.
Biochem/physiol Actions
Collagenase is activated by four gram atom calcium per mole enzyme. It is inhibited by ethylene glycol-bis(beta-aminoethyl ether) - N, N, N′,N′-tetraacetic acid, beta-mercaptoethanol, glutathione, thioglycolic acid and 8-hydroxyquinoline.
The collagenase product is a mixture of enzymes secreted by C. histolyticum, with different products differentiated by the relative ratios of the 10-18 components found in the secreted enzymes. The main components are two collagenases, clostripain, and a neutral protease. The synergistic action of these enzymes degrade collagen and other intracellular materialThe action of both collagenase enzymes and the neutral protease is necessary for effective release of cells from tissue. Various types of collagen are the natural substrates for collagenase.
Caution
As supplied, this product is stable for one year at -20°C. There is no loss in FALGPA or protease activity in 30 days at 37°C, 50°C and -20°C. Solutions of crude collagenase are stable if frozen quickly in aliquots (at 10 mg/mL) and kept frozen at -20°C. Further freeze-thaw cycles will damage the solution. The product retains 100% activity over 7 hours when held on ice.
Unit Definition
One collagen digestion unit (CDU) liberates peptides from collagen from bovine achilles tendon equivalent in ninhydrin color to 1.0 μmole of leucine in 5 hours at pH 7.4 at 37 °C in the presence of calcium ions. One FALGPA hydrolysis unit hydrolyzes 1.0 μmole of furylacryloyl-Leu-Gly-Pro-Ala per min at 25°C. One Neutral Protease unit hydrolyzes casein to produce color equivalent to 1.0 μmole of tyrosine per 5 hr at pH 7.5 at 37°C. One Clostripain Unit hydrolyzes 1.0 μmole of BAEE per min at pH 7.6 at 25°C in the presence of DTT.
Preparation Note
Solutions are typically prepared at 1-2 mg/mL in TESCA buffer (containing 50 mM TES, 0.36 mM Calcium chloride, pH 7.4 at 37°C. This product also contains clostripain, nonspecific neutral protease and tryptic activities.
related product
substrate
Product No.
Description
Pricing
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Resp. Sens. 1 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Zhen-Yu Du et al.
PloS one, 6(6), e20917-e20917 (2011-06-21)
We have developed an in vitro hepatocyte-adipose tissue explant (ATE) co-culture model enabling examination of the effect of visceral and subcutaneous adipose tissues on primary rat hepatocytes. Initial analyses of inflammatory marker genes were performed in fractionated epididymal or inguinal
Sergio Garnica-Galvez et al.
Cells, 10(4) (2021-05-01)
The use of macromolecular crowding in the development of extracellular matrix-rich cell-assembled tissue equivalents is continuously gaining pace in regenerative engineering. Despite the significant advancements in the field, the optimal macromolecular crowder still remains elusive. Herein, the physicochemical properties of
Michael Hajek et al.
Cancers, 13(1) (2020-12-31)
High levels of DNA methylation at CpG loci are associated with transcriptional repression of tumor suppressor genes and dysregulation of DNA repair genes. Human papilloma virus (HPV)-associated head and neck squamous cell carcinomas (HNSCC) have high levels of DNA methylation
Demethylation Therapy as a Targeted Treatment for Human Papillomavirus-Associated Head and Neck Cancer.
Asel Biktasova et al.
Clinical cancer research : an official journal of the American Association for Cancer Research, 23(23), 7276-7287 (2017-09-17)
Israel López-Valero et al.
Theranostics, 10(11), 5120-5136 (2020-04-21)
Glioblastoma (GBM) is one of the most aggressive forms of cancer. It has been proposed that the presence within these tumors of a population of cells with stem-like features termed Glioma Initiating Cells (GICs) is responsible for the relapses that
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service