C4555
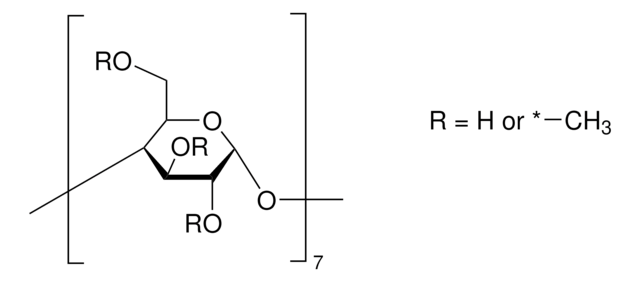
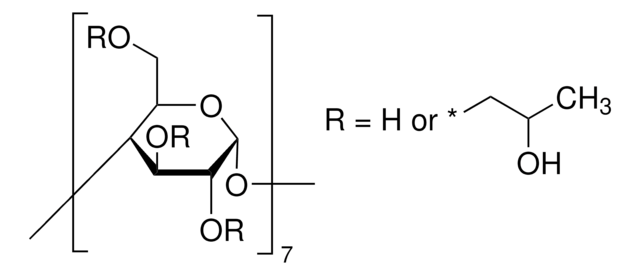
Methyl-β-cyclodextrin
powder, BioReagent, suitable for cell culture
About This Item
Recommended Products
biological source
synthetic (organic)
sterility
non-sterile
product line
BioReagent
form
powder
mol wt
~1320 g/mol
extent of labeling
1.5-2.1 methyl per mol glucose
technique(s)
cell culture | mammalian: suitable
mp
180-182 °C (lit.)
solubility
H2O: 50 mg/mL
storage temp.
room temp
InChI
1S/C56H98O35/c1-64-15-22-36-29(57)43(71-8)50(78-22)86-37-23(16-65-2)80-52(45(73-10)30(37)58)88-39-25(18-67-4)82-54(47(75-12)32(39)60)90-41-27(20-69-6)84-56(49(77-14)34(41)62)91-42-28(21-70-7)83-55(48(76-13)35(42)63)89-40-26(19-68-5)81-53(46(74-11)33(40)61)87-38-24(17-66-3)79-51(85-36)44(72-9)31(38)59/h22-63H,15-21H2,1-14H3
InChI key
QGKBSGBYSPTPKJ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
The solubility of natural cyclodextrins is very poor. In the late 1960′s, it was discovered that chemical substitutions at the 2, 3, and 6 hydroxyl sites would greatly increase solubility. Most chemically modified cyclodextrins are able to achieve a 50% (w/v) concentration in water.
Application
- to study the effect of cholesterol depletion, from sperm membrane on sperm′s ability to undergo acrosome reaction.
- to determine the effect of caveolin overexpression and its loss on pro-survival and pro-growth signaling
- in conventional in vitro fertilization
Biochem/physiol Actions
Reconstitution
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
368.6 °F
Flash Point(C)
187 °C
Personal Protective Equipment
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
How palmitic acid, other fatty acids and other cell culture components affect the performance of serum-free, protein-free cell culture systems used for biomanufacturing heterologous proteins including monoclonal antibodies.
How the unsaturated fatty acid, oleic acid and other cell culture components affect the performance of serum-free, protein-free cell culture systems used for biomanufacturing heterologous proteins including monoclonal antibodies.
Many metabolically important compounds, such as lipid-soluble vitamins and hormones, have very low solubilities in aqueous solutions. Various techniques have been used to solubilize these compounds in tissue culture, cell culture, or other water-based applications.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service