C1141
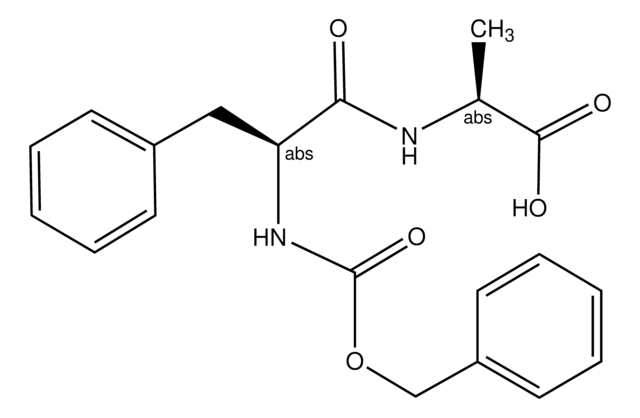
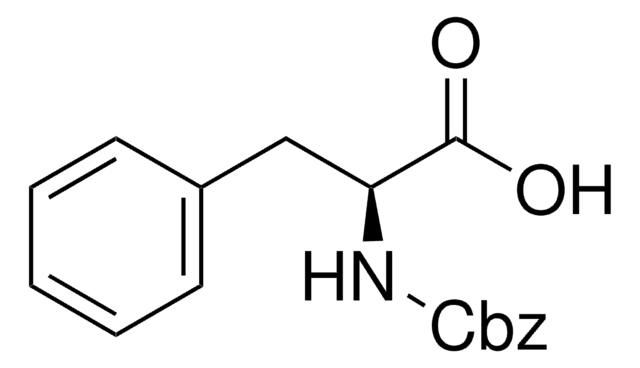
Z-Phe-Leu
≥98% (TLC), suitable for ligand binding assays
Synonym(s):
N-CBZ-Phe-Leu
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C23H28N2O5
CAS Number:
Molecular Weight:
412.48
MDL number:
UNSPSC Code:
12352209
PubChem Substance ID:
NACRES:
NA.26
Recommended Products
Product Name
Z-Phe-Leu,
Assay
≥98% (TLC)
Quality Level
form
powder
technique(s)
ligand binding assay: suitable
color
white
storage temp.
−20°C
SMILES string
CC(C)CC(NC(=O)C(Cc1ccccc1)NC(=O)OCc2ccccc2)C(O)=O
InChI
1S/C23H28N2O5/c1-16(2)13-20(22(27)28)24-21(26)19(14-17-9-5-3-6-10-17)25-23(29)30-15-18-11-7-4-8-12-18/h3-12,16,19-20H,13-15H2,1-2H3,(H,24,26)(H,25,29)(H,27,28)
InChI key
IBOXOGVHBFUSFH-UHFFFAOYSA-N
Amino Acid Sequence
Z-Phe-Leu
Biochem/physiol Actions
N-CBZ-Phe-Leu (Z-phe-Ieu) (CBZ-phenylalanylleucine) is an N-terminal protected Cbz-dipeptide substrate used to differentiate, characterize and kinetically analyze various carboxypeptidase(s).
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
R Raksakulthai et al.
Journal of agricultural and food chemistry, 49(10), 5019-5030 (2001-10-16)
The hepatopancreas of squid (Illex illecebrosus) extract contains a wide range of carboxypeptidase (CP) activities based on hydrolysis of N-CBZ-dipeptide substrates. SDS-PAGE zymograms with N-CBZ-Phe-Leu substrate revealed three activity zones (CP-I, 23 kDa; CP-II, 29 kDa; CP-III, 42 kDa). CP-I
Long-Liu Lin et al.
Journal of biotechnology, 128(2), 322-334 (2006-11-30)
The gene encoding a Deinococcus radiodurans R1 bifunctional aminoacylase/carboxypeptidase (DR_ACY/CP) was amplified by polymerase chain reaction and cloned into pQE-30 to generate pQE-DRAC. The cloned gene consists of an open reading frame of 1197 bp encoding a protein with a
H Ostrowska
Platelets, 8(5), 355-360 (2006-06-24)
Human platelets were investigated for activity of the acidic carboxypeptidases: cathepsin A, lysosomal carboxypeptidase B and prolyl-carboxypeptidase. It was found that the main acidic carboxypeptidase in human platelets had cathepsin A activity. No activity of lysosomal carboxypeptidase B and prolyl-carboxypeptidase
Li Li et al.
Journal of industrial microbiology & biotechnology, 35(1), 41-47 (2007-10-19)
Effects of the enzymes in Actinomucor elegans extract and the enzyme Alcalase 2.4L on debittering the soybean protein hydrolysates were investigated. When the protein was treated only with the latter, a strong bitterness formed; but it decreased if the protein
Joji Mima et al.
European journal of biochemistry, 269(13), 3220-3225 (2002-06-27)
Cys341 of carboxypeptidase Y, which constitutes one side of the solvent-accessible surface of the S1 binding pocket, was replaced with Gly, Ser, Asp, Val, Phe or His by site-directed mutagenesis. Kinetic analysis, using Cbz-dipeptide substrates, revealed that polar amino acids
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service