Y0001554
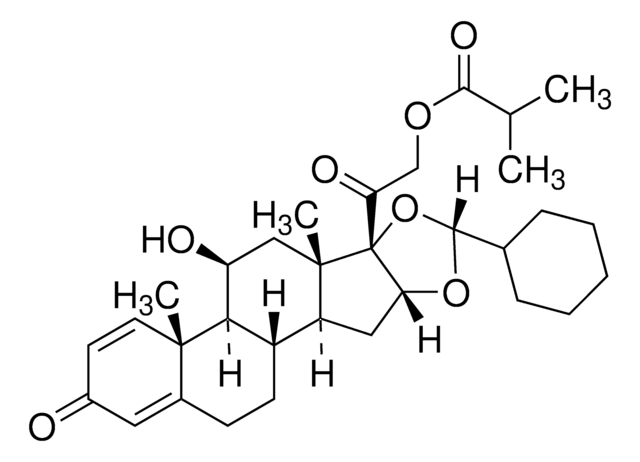
Ciclesonide containing impurity A
European Pharmacopoeia (EP) Reference Standard
About This Item
Recommended Products
biological source
synthetic
grade
pharmaceutical primary standard
Agency
EP
API family
ciclesonide
packaging
pkg of 15 mg
manufacturer/tradename
EDQM
storage condition
protect from light
color
white
bp
665.0 °C/1.333 hPa (1229.0°F)
mp
209-211 °C (408—412°F)
solubility
water: <0.1 g/L
acetone: soluble
ethanol: soluble
density
1.23 g/cm3 at 20 °C (1.333 hPa)
application(s)
pharmaceutical (small molecule)
format
neat
shipped in
ambient
storage temp.
2-8°C
General description
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets, have been developed and issued under the Authority of the issuing Pharmacopoeia. For further information and support please go to the website of the issuing Pharmacopoeia.
Application
Packaging
Other Notes
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Sorry, we don't have COAs for this product available online at this time.
If you need assistance, please contact Customer Support.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service