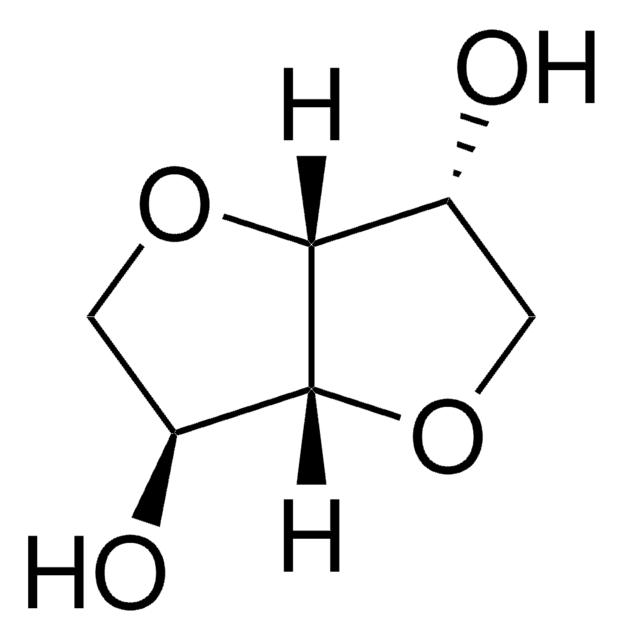
I0775020
Isosorbide 2-nitrate
European Pharmacopoeia (EP) Reference Standard
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C6H9NO6
CAS Number:
Molecular Weight:
191.14
UNSPSC Code:
41116107
NACRES:
NA.24
Recommended Products
grade
pharmaceutical primary standard
API family
isosorbide
manufacturer/tradename
EDQM
application(s)
pharmaceutical (small molecule)
format
neat
InChI
1S/C6H9NO6/c8-3-1-11-6-4(13-7(9)10)2-12-5(3)6/h3-6,8H,1-2H2/t3-,4+,5-,6-/m1/s1
InChI key
YWXYYJSYQOXTPL-JGWLITMVSA-N
Looking for similar products? Visit Product Comparison Guide
General description
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the Issuing Pharmacopoeia. For further information and support please go to the website of the issuing Pharmacopoeia.
Application
Isosorbide 2-nitrate EP Reference standard, intended for use in laboratory tests only as specifically prescribed in the European Pharmacopoeia.
Packaging
The product is delivered as supplied by the issuing Pharmacopoeia. For the current unit quantity, please visit the EDQM reference substance catalogue.
Other Notes
Sales restrictions may apply.
related product
Product No.
Description
Pricing
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Lot/Batch Number
Sorry, we don't have COAs for this product available online at this time.
If you need assistance, please contact Customer Support.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
D Smith et al.
Drug metabolism and disposition: the biological fate of chemicals, 18(4), 429-434 (1990-07-01)
A pharmacokinetic model is proposed to describe the plasma levels of isosorbidedinitrate (ISDN) and its two pharmacologically active metabolites, isosorbide-2-mononitrate (IS-2MN) and isosorbide-5-mononitrate (IS-5MN), following the oral administration of several 20-mg sustained release formulations of ISDN. Absorption of ISDN from
T Murakawa et al.
Masui. The Japanese journal of anesthesiology, 42(2), 225-232 (1993-02-01)
Nineteen patients with ischemic heart disease were studied to determine plasma levels of isosorbide dinitrate (ISDN) and its metabolites, isosorbide-2-mononitrate (2-ISMN) and isosorbide-5-mononitrate (5-ISMN) for 6 hrs during intravenous administration of ISDN, using gas chromatography. Differences in plasma levels of
V Hutt et al.
Arzneimittel-Forschung, 43(8), 842-846 (1993-08-01)
In the course of this study the bioavailability and pharmacokinetic profile of a newly developed 2.5 mg (per valve release) oral isosorbide dinitrate (ISDN, CAS 87-33-2) spray preparation (Isoket Spray) were determined and compared with the results for an already
W Schneider et al.
European journal of clinical pharmacology, 38(2), 145-147 (1990-01-01)
The concentrations of isosorbide dinitrate (ISDN), isosorbide-5-mononitrate (IS-5-MN) and isosorbide-2-mononitrate (IS-2-MN) were determined in plasma (PL), saphenous vein wall (SV) and pectoral muscle (PM) from 8 patients undergoing coronary bypass surgery. The patients were pretreated for 2 days with ISDN
M Diestelhorst et al.
International ophthalmology, 15(4), 259-262 (1991-07-01)
In a randomized, double-masked, single drop study the effect of topical isosorbid-mononitrate (ISMO) 0.5% eye drops, the main metabolite of isosorbid-dinitrate, widely used in the treatment of coronary heart diseases, was studied in patients suffering from open angle glaucoma or
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service

![(3S,3aS,6R,6aR)-6-hydroxyhexahydrofuro[3,2-b]furan-3-yl nitrate AldrichCPR](/deepweb/assets/sigmaaldrich/product/structures/381/270/d8615042-7a4c-4eb0-b24b-70f9841deb64/640/d8615042-7a4c-4eb0-b24b-70f9841deb64.png)