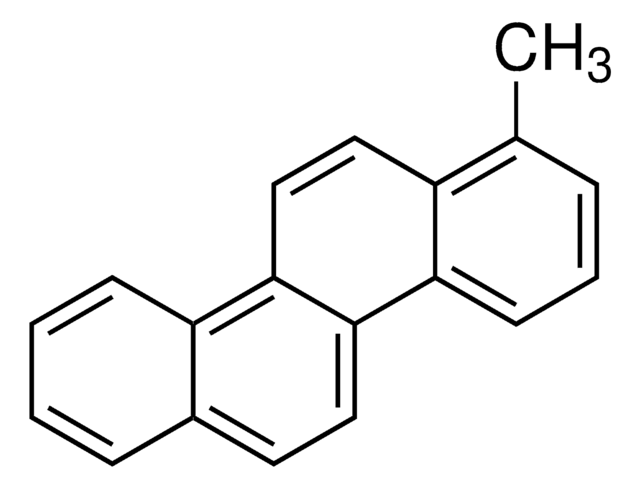
BCR137R
Benzo[b]naphtho[1,2-d]thiophene
BCR®, certified reference material
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C16H10S
CAS Number:
Molecular Weight:
234.32
Beilstein:
9635
MDL number:
UNSPSC Code:
41116107
PubChem Substance ID:
NACRES:
NA.24
Recommended Products
grade
certified reference material
Agency
BCR®
manufacturer/tradename
JRC
technique(s)
HPLC: suitable
gas chromatography (GC): suitable
format
neat
storage temp.
2-8°C
SMILES string
c1ccc2c(c1)ccc3sc4ccccc4c23
InChI
1S/C16H10S/c1-2-6-12-11(5-1)9-10-15-16(12)13-7-3-4-8-14(13)17-15/h1-10H
InChI key
XZUMOEVHCZXMTR-UHFFFAOYSA-N
Analysis Note
For more information please see:
BCR137R
BCR137R
Legal Information
BCR is a registered trademark of European Commission
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Lot/Batch Number
Sorry, we don't have COAs for this product available online at this time.
If you need assistance, please contact Customer Support.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Jose Juan Rodríguez et al.
Organic & biomolecular chemistry, 10(36), 7334-7346 (2012-08-01)
Based on the benzo[b]naphtho[1,2-d]furan and benzo[b]naphtho[1,2-d]thiophene frameworks, a series of ligands with different basic side chains (BSCs) has been synthesized and pharmacologically evaluated. Also, their binding modes have been modelled using docking techniques. It was found that the introduction of
J Jacob et al.
Cancer letters, 32(1), 107-116 (1986-07-01)
Thiaarenes are metabolized by liver microsomes of untreated rats predominantly to sulfones and sulfoxides. After pretreatment of rats with monooxygenase inducers, ring oxidation of thiaarenes is also observed. In case of benzo[b]naphtho[2,3-d]thiophene the formation of a p-quinone takes place. Rat
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
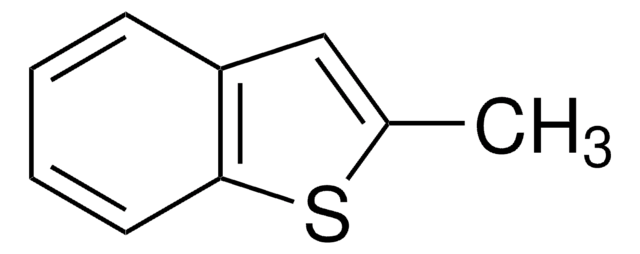
Contact Technical Service![Benzo[b]naphtho[2,3-d]thiophene BCR®, certified reference material](/deepweb/assets/sigmaaldrich/product/structures/232/635/832d9c75-8dd4-4be6-8808-ac1356cf128f/640/832d9c75-8dd4-4be6-8808-ac1356cf128f.png)
![Benzo[b]thien-2-ylboronic acid ≥95%](/deepweb/assets/sigmaaldrich/product/structures/251/077/d0ead874-b533-4dcb-890d-8816a0018ccd/640/d0ead874-b533-4dcb-890d-8816a0018ccd.png)
![Dibenzo[b,d]furan BCR®, certified reference material](/deepweb/assets/sigmaaldrich/product/structures/826/318/6071deff-c217-4b9e-916b-24a0ce6d3b83/640/6071deff-c217-4b9e-916b-24a0ce6d3b83.png)