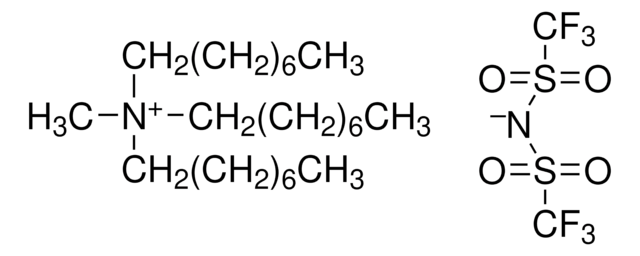86838
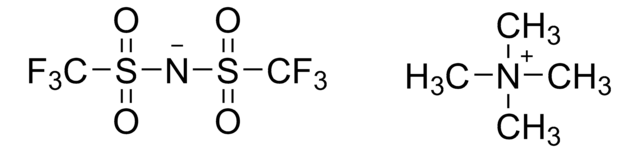
Tetrabutylammonium bis-trifluoromethanesulfonimidate
for electronic purposes, ≥99.0%
About This Item
Recommended Products
grade
for electronic purposes
Quality Level
Assay
≥99.0% (qNMR)
≥99.0%
form
crystals
mp
90-95 °C
SMILES string
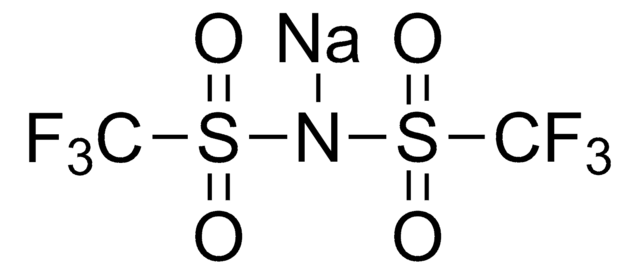
FC(F)(F)S(=O)(=O)[N-]S(=O)(=O)C(F)(F)F.CCCC[N+](CCCC)(CCCC)CCCC
InChI
1S/C16H36N.C2F6NO4S2/c1-5-9-13-17(14-10-6-2,15-11-7-3)16-12-8-4;3-1(4,5)14(10,11)9-15(12,13)2(6,7)8/h5-16H2,1-4H3;/q+1;-1
InChI key
CFAPFDTWIGBCQK-UHFFFAOYSA-N
General description
Application
- The cohesive properties and pyrolysis mechanism of an aprotic ionic liquid tetrabutylammonium bis(trifluoromethanesulfonyl)imide.: This study investigates the cohesive properties and thermal decomposition mechanism of tetrabutylammonium bis(trifluoromethanesulfonyl)imide, providing insights into its stability and potential applications in analytical and materials chemistry (Liu et al., 2023).
Other Notes
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Dam. 1 - Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Over the past decade, Ionic Liquids have attracted much interest for their use as non-aqueous electrolytes in electrochemical applications. In this context, their conductivity as well as their electrochemical stability are the most important physical properties.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service