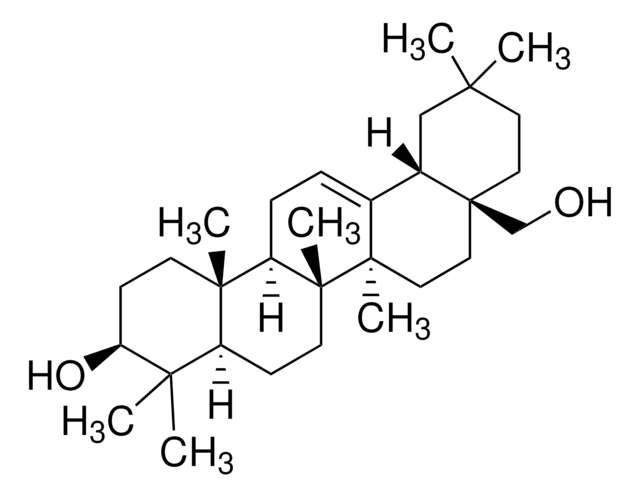
09236
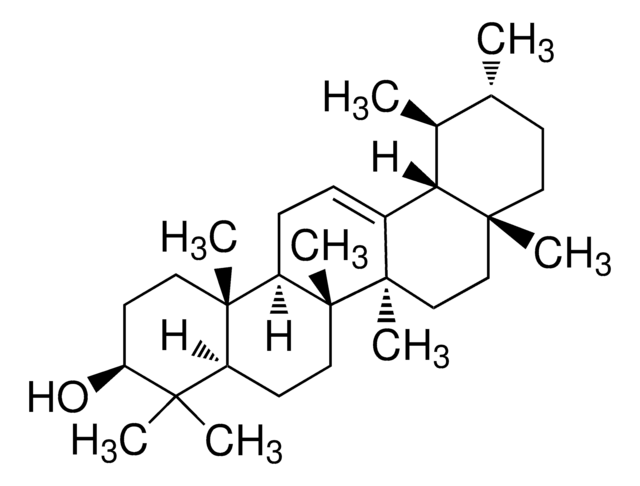
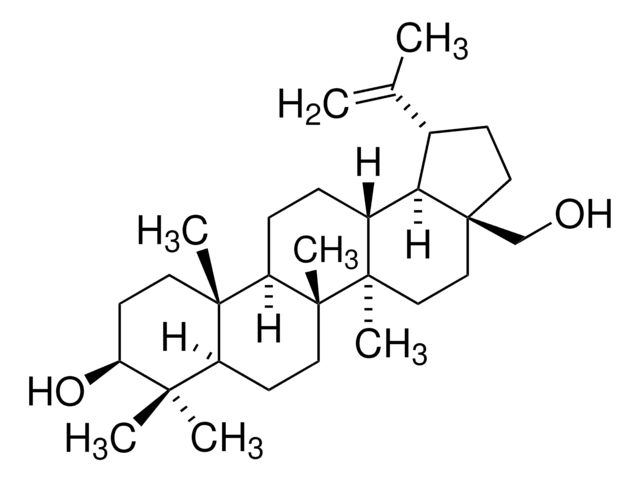
β-Amyrin
analytical standard
Synonym(s):
β-Amyrenol, Olean-12-en-3β-ol
About This Item
Recommended Products
grade
analytical standard
Quality Level
Assay
≥98.5% (HPLC)
shelf life
limited shelf life, expiry date on the label
technique(s)
HPLC: suitable
gas chromatography (GC): suitable
application(s)
food and beverages
format
neat
SMILES string
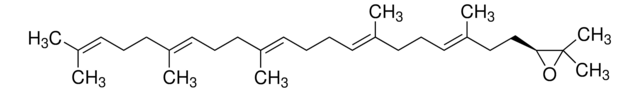
[H][C@@]12CC(C)(C)CC[C@]1(C)CC[C@]3(C)C2=CC[C@]4([H])[C@@]5(C)CC[C@H](O)C(C)(C)[C@]5([H])CC[C@@]34C
InChI
1S/C30H50O/c1-25(2)15-16-27(5)17-18-29(7)20(21(27)19-25)9-10-23-28(6)13-12-24(31)26(3,4)22(28)11-14-30(23,29)8/h9,21-24,31H,10-19H2,1-8H3/t21-,22-,23+,24-,27+,28-,29+,30+/m0/s1
InChI key
JFSHUTJDVKUMTJ-QHPUVITPSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Application
- Development and validation of two high-performance thin layer chromatography (HPTLC) methods to quantify four biomarkers— β-amyrin, β-sitosterol, lupeol, and ursolic acid in the ethanol extract of G. senegalensis leaves
- Analysis of methanol extracts of leaf samples collected from five Ficus species by a novel HPTLC densitometric method, for the determination of β-amyrin, validated according to the International Conference on Harmonization (ICH) guidelines
- Quantification of β-amyrin in the ethanolic extracts collected from two Maytenus species, M. obscura and M. parviflora by a validated HPTLC-densitometric method
- Secondary metabolite profiling of various plant parts collected from 82 plants belonging to 21 different cannabis strains using gas chromatography-mass spectrometry (GC-MS) for sterols and terpenoids (mono-, sesqui-, tri-), and high-performance liquid chromatography (HPLC) with UV and mass spectrometric (MS) detection for flavonoids
Biochem/physiol Actions
Packaging
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Terpenes comprise the largest and most diverse class of secondary metabolites; approximately 55,000 compounds have been identified to date.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service