SLGPM33RS
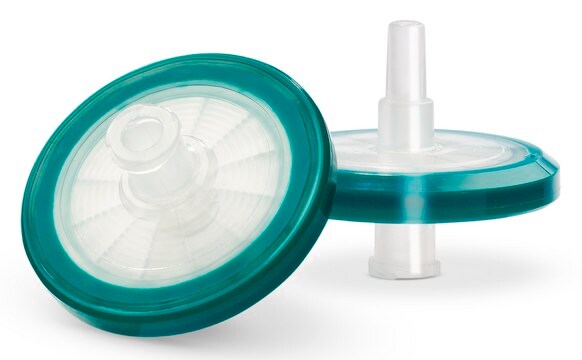
Millex®-GP Filter Unit (Sterile)
pore size 0.22 μm, diam. 33 mm, polyethersulfone membrane, hydrophilic, medical device
Synonym(s):
disposable syringe filter, polyethersulfone syringe filter, sterile syringe filter, syringe filter
About This Item
Recommended Products
material
modified acrylic housing
polyethersulfone membrane
Quality Level
sterility
sterile; γ-irradiated
product line
Millex®
feature
holdup volume≤ 0.1 mL
hydrophilic
FDA registered
medical device
manufacturer/tradename
Millex®
parameter
10.3 bar max. inlet pressure (150 psi)
45 °C max. temp.
diam.
33 mm
filtration area
4.52 cm2
inlet to outlet H
27 mm
volume
200 mL
pore size
0.22 μm pore size
fitting
female Luer-Lok® inlet
male Luer outlet slip
shipped in
ambient
General description
Application
- Clinical benefits: Removal of microorganisms, particles, precipitates, and undissolved powders larger than the membrane’s rated pore size from solutions for clinical use.
- Hospital pharmacy: Sterile filtration and/or clarification of small volumes of protein pharmaceuticals, diagnostic imaging agents, chemotherapeutics, aqueous solutions, or water during admixture preparation.
- Direct patient care: Sterilization and/or particulate removal from epidural and other liquid anaesthetics as well as from irrigation solutions used in ophthalmic, otic, and other surgical procedures.
Features and Benefits
- Bidirectional support of the filter membrane
- Removes microorganisms, particles, precipitates, and undissolved powders larger than the membrane′s rated pore size
- Single-use, non-pyrogenic and non-toxic
- Polyethersulfone membrane filter sealed in a modified acrylic copolymer (MMA) housing
- Compatible with most aqueous solutions
- CE marked and FDA registered
Other Notes
Legal Information
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service