MABN878
Anti-phospho-Amyloid beta (Ser8) Antibody, clone 1E4E11
clone 1E4E11, from mouse
Synonym(s):
Aβ peptide, Ser8 phosphorylated, Abeta peptide, Ser8 phosphorylated, Amyloid β peptide, Ser8 phosphorylated, Aβ1-40, Ser8 phosphorylated, Aβ1-42, Ser8 phosphorylated, Abeta1-40, Ser8 phosphorylated, Abeta1-42, Ser8 phosphorylated
About This Item
Recommended Products
biological source
mouse
Quality Level
antibody form
purified immunoglobulin
antibody product type
primary antibodies
clone
1E4E11, monoclonal
species reactivity
human
technique(s)
ELISA: suitable
immunofluorescence: suitable
immunohistochemistry: suitable
western blot: suitable
isotype
IgG1κ
NCBI accession no.
UniProt accession no.
target post-translational modification
phosphorylation (pSer8)
Gene Information
human ... APP(351)
General description
Specificity
Immunogen
Application
Neuroscience
Neurodegenerative Diseases
Western Blotting Analysis: 2 µg/mL from a representative lot detected phosphorylated oligomeric amyloid beta (pAβ) peptides in 50 µg of brain extract from an 8-month old APP/PS1KI transgenic mouse, but not in extract from an age-matched non-transgenic mouse (Courtesy of Dr. Kumar and Prof. Dr. Walter, Department of Neurology, University Bonn, Germany).
ELISA Analysis: Clone 1E4E11 hybridoma culture supernatant detected the immunogen peptide with phosphorylated Ser8, but not the corresponding non-phosphorylated peptide (Kumar, S., et al. (2013). Acta Neuropathol. 125(5):699-709).
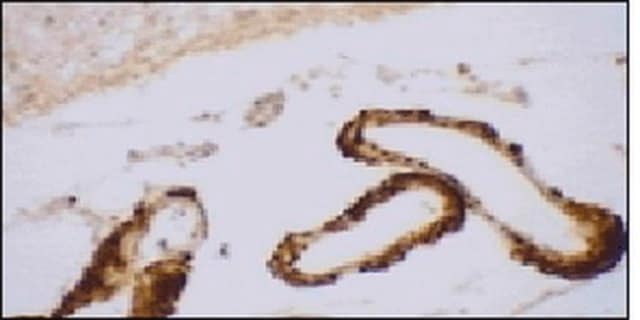
Immunohistochemistry Analysis: A representative lot detected age-dependent phospho-amyloid beta (pAβ) immunoreactivity and localization in paraffin-embedded APP/PS1KI mouse brain sections following antigen retrieval by heat and 88% formic acid treatments (Kumar, S., et al. (2013). Acta Neuropathol. 125(5):699-709).
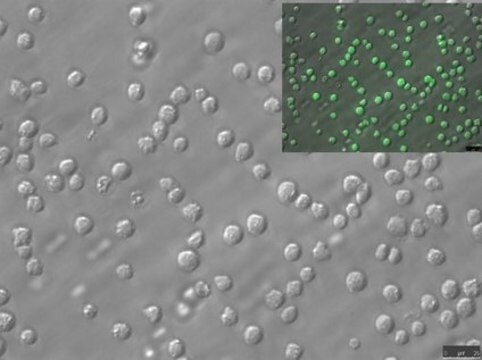
Immunofluorescence Analysis: A representative lot detected phospho-amyloid beta (pAβ) immunoreactivity in cortical neurons colocalized with that detected with a non-phospho-specific Aβ antibody by dual fluorescent immunohistochemistry staining of paraffin-embedded 2-month old APP/PS1KI mice cotex sections following anigen retrieval by heat and 88% formic acid treatments (Kumar, S., et al. (2013). Acta Neuropathol. 125(5):699-709).
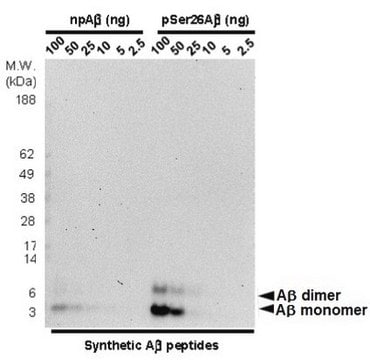
Western Blotting Analysis: A representative lot detected monomeric, dimeric, trimeric, and oligomeric forms of synthetic Aβ1-40 and Aβ1-42 peptides with phosphorylated Ser8, but not the non-phosphorylated Aβ1-40 and Aβ1-42 peptides (Kumar, S., et al. (2013). Acta Neuropathol. 125(5):699-709).
Western Blotting Analysis: A representative lot detected monomeric, dimeric, and oligomeric forms of phosphorylated amyloid beta (pAβ) peptides in brain extracts from 6- and 12-month old APP/PS1KI transgenic mice, but not in brain extracts from age-matched non-transgenic mice (Kumar, S., et al. (2013). Acta Neuropathol. 125(5):699-709).
Note: Formic acid (88%) treatment following heat retrieval is recommended for immunohistochemical detection of aggregated intraneuronal Abeta peptides in brain sections (Kumar, S., et al. (2013). Acta Neuropathol. 125(5):699-709; Christensen, D.Z., et al. (2009). Brain Res. 1301:116-125).
Quality
Isotyping Analysis: The identity of this monoclonal antibody is confirmed by isotyping test to be IgG1κ.
Target description
Physical form
Storage and Stability
Other Notes
Disclaimer
Not finding the right product?
Try our Product Selector Tool.
Storage Class Code
12 - Non Combustible Liquids
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service