MAB305
Anti-Choline Acetyltransferase Antibody, clone 1E6
ascites fluid, clone 1E6, Chemicon®
Synonym(s):
ChAT, Choline Acetylase, CHOACTase
About This Item
Recommended Products
biological source
mouse
Quality Level
antibody form
ascites fluid
antibody product type
primary antibodies
clone
1E6, monoclonal
species reactivity
human, rat, monkey
manufacturer/tradename
Chemicon®
technique(s)
immunohistochemistry: suitable
isotype
IgG1
NCBI accession no.
UniProt accession no.
shipped in
dry ice
target post-translational modification
unmodified
Gene Information
human ... CHAT(1103)
rat ... Chat(290567)
rhesus monkey ... Chat(709977)
Specificity
Immunogen
Application
Optimal working dilutions must be determined by the end user.
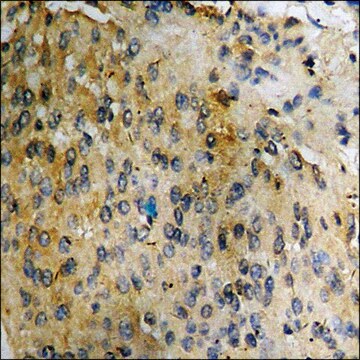
IMMUNOHISTOCHEMISTRY PROCEDURE (PAP TECHNIQUE) FOR MAB305, MONOCLONAL ANTIBODY TO CHOLINE ACETYLTRANSFERASE
I) Perfusion & Sectioning Procedure
1. Perfuse through the heart with a fixative solution containing 4% paraformaldehyde in 0.12 M phosphate buffer (pH 7.3) for light microscopy (LM), and additionally, 0.1% gluteraldehyde and .002% CaCl2 for electron microscopy (EM).
2. Remove brain and postfix 2-18 hours at 4°C in 4% paraformaldehyde in 0.12 M phosphate buffer.
3. After brain is blocked for sectioning, wash in several changes of buffer for 2-3 hours.
4. Specimens for EM are sectioned on a Vibratome (50 μm) and rinsed in buffer, those for LM should be cryoprotected in 30% sucrose in buffer.
5. After freezing with dry ice, 30-40 μm thick sections of LM specimens are cut on a cryostat.
6. Sections are rinsed, and then stored in phosphate buffer containing 0.1% sodium azide.
II) Staining Procedure
Tissue is processed as freely-floating sections in continuously agitated solutions. All incubations are performed at room temperature unless otherwise stated.
1.a. For localizing ChAT-positive somata and dendrites:
Sections are washed in 0.1 M Tris-buffered saline (TBS; containing 1.4% NaCl, pH 7.3) only. No detergent or enzyme pretreatment is used.
b. For localizing ChAT-positive terminal-like structures:
Incubate sections in TBS (pH 8.1) for 5 minutes at 37°C. Transfer sections to TBS (pH 8.1) containing pronase (1.2 μg/mL) for 1 1/2-2 minutes at 37°C, followed by several ice cold buffer washes for a total of 5 minutes. The concentration of pronase and incubation time of the digestion should be evaluated for each region examined.
c. For localizing ChAT immunoreactivity and subsequently counterstaining the sections:
Incubation in TBS containing 0.1%-0.8% Triton X-100 for 15 minutes may increase the tissue penetration of the immunoreagents, but it also raises the background staining.
2. Incubate sections in normal goat serum (3-5%) for one hour. The working solutions of all antisera should also contain similarly diluted normal goat serum.
3. Incubate in anti-ChAT monoclonal antibody solution (Suggested working dilution 1:250, final working dilution must be determined by end user) for 2 hours at room temperature and then for an additional 6-18 hours at 4°C.
4. Incubate with second antibody (i.e. Goat anti-Mouse IgG, Cat. No.: AP124, dilution 1:50-100) for 1-2 hours.
5. Incubate with diluted PAP complex (i.e. Mouse PAP, Cat No.: PAP14, conc. 25-50 μg/mL) for one hour.
6. After rinsing in buffer, the second antibody and PAP steps are repeated for 40 minutes to 1 hour each in order to amplify staining intensity, particularly of small ChAT-containing structures.
7. React for 15 minutes with 0.06% 3,3′-diaminobenzidine×4 HCl (DAB; diluted in phosphate buffered saline, pH 7.3) and 0.006% H2O2.
8. Specimens for routine LM are postfixed for 1 minutes in 0.005% OsO4 (osmium tetraoxide), and then mounted, dehydrated and coverslipped. Selected regions blocked for EM are postfixed in 2% OsO4 for 1 hour, en bloc stained with uranyl acetate, and flat-embedded in Epon-Araldite resin.
Neuroscience
Neurotransmitters & Receptors
Neuronal & Glial Markers
Physical form
Storage and Stability
Analysis Note
Brain tissue
Other Notes
Legal Information
Disclaimer
Not finding the right product?
Try our Product Selector Tool.
Storage Class Code
10 - Combustible liquids
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service